Also see:
Endometriosis and Estrogen
Hormonal profiles in women with breast cancer
A Physiological Approach to Ovarian Cancer
Ray Peat, PhD on the Menstrual Cycle
Breast Cancers Can Produce Their Own Estrogen to Resist Aromatase Inhibitors
“After menopause, estrogen is produced less by the ovary than by other tissues, including fat cells. Breast cancer cells can produce their own estrogen. This means that estrogen is one of the “cancer hormones” which, secreted by the tumor, promotes the growth of the tumor and also has a systemic pro-cancer action. Ovarian tumors, too, can cause great systemic hormonal imbalances, which should be investigated routinely in a physiological approach to the disease. After menopause, estrogen is largely produced by conversion of “androgenic” steroids from the adrenal glands. It would be desirable to be able to inhibit the conversion of the adrenal steroid precursor to estrogen. Newton, et al.(11) showed that progesterone inhibits the conversion of the precursor to estrogen, and P.K. Siiteri showed a similar effect for thyroid hormone.” -Ray Peat, PhD
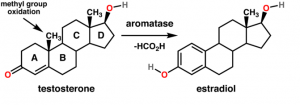
http://en.wikipedia.org/wiki/File:Testosterone_estradiol_conversion.png
J Steroid Biochem. 1986 May;24(5):1033-9.
Aromatase activity and concentrations of cortisol, progesterone and testosterone in breast and abdominal adipose tissue.
Newton CJ, Samuel DL, James VH.
Aromatase activity and concentrations of cortisol, progesterone and testosterone were measured in samples of breast and abdominal adipose tissue obtained from both pre- and postmenopausal subjects. Enzyme activity was determined by the incorporation of tritium from [1 beta-3H]androstenedione into water and found to be in close agreement to that measured when tritium labelled oestrone (E1) and oestradiol (E2) were isolated. No significant difference in enzyme activity was noted between breast and abdominal adipose tissue. Increased aromatase activity was not observed in adipose tissue taken from a subject with endometrial cancer. Cortisol concentrations were found to be significantly higher (P less than 0.05) in abdominal as compared to breast tissue. Without attaining statistical significance progesterone concentrations were higher in abdominal as compared to breast adipose tissue. Aromatase activity was not related to either cortisol or testosterone tissue concentration, but an inverse relationship between progesterone concentration and aromatase activity was observed (r = 0.542, P less than 0.02). On the basis of results obtained a hypothesis for the increased conversion of androgen to oestrogen as seen after the menopause has been proposed.
===========================================
Aromatase and Estrogen’s Role in Cancer of the Breast:
Eur J Cancer Clin Oncol. 1986 Apr;22(4):515-25.
Aromatase, 17 beta-hydroxysteroid dehydrogenase and intratissular sex hormone concentrations in cancerous and normal glandular breast tissue in postmenopausal women.
Vermeulen A, Deslypere JP, Paridaens R, Leclercq G, Roy F, Heuson JC.
In a study of the origin of estrogens in patients with breast cancer, the concentrations of estrogens and their androgen precursors, and aromatase and 17 beta-hydroxysteroid dehydrogenase (E2DH) activities were determined in normal glandular and cancerous breast tissue. The correlation between tissue estrogens, precursor concentrations, enzyme activities and plasma levels and/or receptor status were calculated. In both normal glandular and carcinomatous breast tissue, the concentrations of androstenedione (A), dehydroepiandrosterone (DHEA), 5 androstene-3 beta, 17 beta-diol (5-Adiol), estrone (E1), estradiol (E2) and progesterone (P) were significantly higher than plasma concentrations. While testosterone (T) concentrations were similar, dehydroepiandrosterone (DHCA) and estrone sulphate (E1S) concentrations were lower in tissue than in plasma. In carcinomatous tissue androgen concentrations were lower, but estrogen concentrations were higher than in glandular breast tissue. Estradiol (E2) concentration was positively correlated with the receptor concentration with the mean E2 concentration corresponding to an estimated receptor occupancy of about 25%, probably sufficient for a submaximal biological response. Aromatase and E2DH (E2—-E1) activities were observed in all breast cancer and glandular breast tissues, activities being higher in carcinoma than in glandular breast tissues; nevertheless, aromatase activity accounts probably only for a small fraction of tissue estrogen concentration. E2DH, but not aromatase activity, was significantly higher in estrogen receptor positive than in estrogen receptor negative tissues and was negatively correlated with tissue dehydroepiandrosterone (DHEA) and its sulphate (DHEAS) concentration; the latter two steroids are non competitive inhibitors of E2DH which inactivates E2 to E1. This effect of DHEA(S) may constitute a mechanism by which these androgens stimulate cancer growth and a rationale (besides suppression of estrogen precursors) for medical or surgical adrenalectomy in hormone sensitive metastatic mammary cancer. E2DH activity might constitute an additional marker of hormone dependency of mammary cancer.
Endocr Relat Cancer June 1, 1999 6 307-314
Aromatase overexpression and breast hyperplasia, an in vivo model–continued overexpression of aromatase is sufficient to maintain hyperplasia without circulating estrogens, and aromatase inhibitors abrogate these preneoplastic changes in mammary glands.
R R Tekmal, N Kirma, K Gill and K Fowler
To test directly the role of breast-tissue estrogen in initiation of breast cancer, we have developed the aromatase-transgenic mouse model and demonstrated for the first time that increased mammary estrogens resulting from the overexpression of aromatase in mammary glands lead to the induction of various preneoplastic and neoplastic changes that are similar to early breast cancer. Continued overexpression of aromatase that leads to increased breast-tissue estrogen contributes to a number of epigenetic changes in mammary tissue such as alteration in the regulation of genes involved in apoptosis, activation of genes involved in cell cycle and cell proliferation, and activation of a number of growth factors. Our current studies show aromatase overexpression is sufficient to induce and maintain early preneoplastic and neoplastic changes in female mice without circulating ovarian estrogen. Preneoplastic and neoplastic changes induced in mammary glands as a result of aromatase overexpression can be completely abrogated with the administration of the aromatase inhibitor, letrozole. Consistent with complete reduction in hyperplasia, we have also seen downregulation of estrogen receptor and a decrease in cell proliferation markers, suggesting aromatase-induced hyperplasia can be treated with aromatase inhibitors. Our studies demonstrate that aromatase overexpression alone, without circulating estrogen, is responsible for the induction of breast hyperplasia and these changes can be abrogated using aromatase inhibitors.
Mol Endocrinol. 2008 Aug;22(8):1812-24. Epub 2008 May 15.
Progesterone receptor inhibits aromatase and inflammatory response pathways in breast cancer cells via ligand-dependent and ligand-independent mechanisms.
Hardy DB, Janowski BA, Chen CC, Mendelson CR.
Aromatase (product of CYP19 gene), the critical enzyme in estrogen biosynthesis, is up-regulated in 70% of all breast cancers and is highly correlated with cyclooxygenase 2 (COX-2), the rate-determining enzyme in prostanoid biosynthesis. Expression of COX-2 also is correlated with the oncogene HER-2/neu. The efficacy of current endocrine therapies for breast cancer is predicted only if the tumor contains significant amounts of estrogen receptor. Because the progesterone receptor (PR) is an estrogen-induced target gene, it has been suggested that its presence may serve as an indicator of estrogen receptor functional capacity and the differentiation state of the tumor. In the present study, we tested the hypothesis that PR serves a crucial protective role by antagonizing inflammatory response pathways in the breast. We observed that progesterone antagonized the stimulatory effects of cAMP and IL-1beta on aromatase, COX-2, and HER-2/neu expression in T47D breast cancer cells. These actions of progesterone were associated with increased expression of the nuclear factor-kappaB inhibitor, IkappaBalpha. In 28 breast cancer cell lines, IkappaBalpha expression was positively correlated with PR mRNA levels; overexpression of a phosphorylation-defective mutant of IkappaBalpha inhibited expression of aromatase, COX-2, and HER-2/neu. Moreover, in breast cancer cell lines cultured in the absence of progesterone, up-regulation of endogenous PR caused decreased expression of aromatase, COX-2, and HER-2/neu expression, whereas down-regulation of endogenous PR resulted in a marked induction of aromatase and HER-2/neu mRNA. Collectively, these findings suggest that PR plays an important antiinflammatory role in breast cancer cells via ligand-dependent and ligand-independent mechanisms.
J Endocrinol 1998 Sep; 158(3):40 1-7.
Progesterone inhibits glucocorticoid-dependent aromatase induction in human adipose fibroblasts.
Schmidt M, Renner C, Loffler G
In fibroblasts derived from human adipose tissue, aromatase induction is observed after exposure to 1 microM cortisol in the presence of serum or platelet-derived growth factor (PDGF). Progesterone suppresses this induction in a dose-dependent manner, 10 microM resulting in complete inhibition. A reduced cortisol concentration (0.1 microM) concomitantly reduces the progesterone concentration required for effective inhibition (10-100 nM). This effect of progesterone is specific, as neither the release of cellular enzymes nor aromatase induction by dibutyryl-cAMP, which acts independently from cortisol, are affected. However, the inhibitory effect of progesterone requires its presence throughout the induction period. Kinetic studies in intact cells reveal a reduced number of aromatase active sites upon progesterone treatment, whereas progesterone at near-physiological concentration (100 nM) does not inhibit aromatase activity in isolated microsomes. Semi-quantitative reverse transcriptase PCR analysis shows reduced amounts of aromatase mRNA in progesterone-treated cells, indicating specific inhibition of the glucocorticoid-dependent pathway of aromatase induction. The inhibitory effect of progesterone is not blocked by the anti-progestin ZK114043, excluding action via progesterone receptors and indicating competition for the glucocorticoid receptor. Progesterone must be considered a potential physiological inhibitor of glucocorticoid-dependent aromatase induction in adipose tissue. It is proposed that it is a suppressor of aromatase induction in adipose tissue in premenopausal women.
Nat Genet. 2017 Jan 23. doi: 10.1038/ng.3773. [Epub ahead of print]
Acquired CYP19A1 amplification is an early specific mechanism of aromatase inhibitor resistance in ERα metastatic breast cancer.
Magnani L, Frigè G, Gadaleta RM, Corleone G, Fabris S, Kempe H, Verschure PJ, Barozzi I, Vircillo V, Hong SP, Perone Y, Saini M, Trumpp A, Viale G, Neri A, Ali S, Colleoni MA11, Pruneri G, Minucci S
Tumor evolution is shaped by many variables, potentially involving external selective pressures induced by therapies. After surgery, patients with estrogen receptor (ERα)-positive breast cancer are treated with adjuvant endocrine therapy, including selective estrogen receptor modulators (SERMs) and/or aromatase inhibitors (AIs). However, more than 20% of patients relapse within 10 years and eventually progress to incurable metastatic disease. Here we demonstrate that the choice of therapy has a fundamental influence on the genetic landscape of relapsed diseases. We found that 21.5% of AI-treated, relapsed patients had acquired CYP19A1 (encoding aromatase) amplification (CYP19A1amp). Relapsed patients also developed numerous mutations targeting key breast cancer-associated genes, including ESR1 and CYP19A1. Notably, CYP19A1amp cells also emerged in vitro, but only in AI-resistant models. CYP19A1 amplification caused increased aromatase activity and estrogen-independent ERα binding to target genes, resulting in CYP19A1amp cells showing decreased sensitivity to AI treatment. These data suggest that AI treatment itself selects for acquired CYP19A1amp and promotes local autocrine estrogen signaling in AI-resistant metastatic patients.

Interesting to know that glyphosate can act in a positive manner to reduce aromatase! Also, the fact that thyroid hormone inhibits aromatase may account for the high levels of NIS (the Na+/I- symporter) present in cancerous breast tissue as the body tries vainly to control the over-expression of estrogen via thyroid hormone. Now on to discover if I3C has an inhibitory mechanism here with its effects on ratios of estrogens or even estriol from flax seed lignans which might block reception of stronger estrogens.