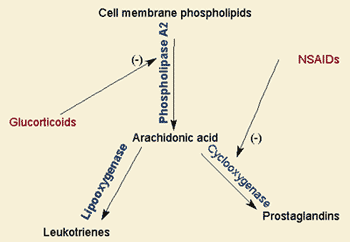
Also see:
Unsaturated Fats and Lung Function
Benefits of Aspirin
Arachidonic Acid’s Role in Stress and Shock
Dietary PUFA Reflected in Human Subcutaneous Fat Tissue
Toxicity of Stored PUFA
PUFA, Fish Oil, and Alzheimers
PUFA – Accumulation and Aging
Brain Swelling Induced by Polyunsaturated Fats (PUFA)
Fish Oil Toxicity
Estrogen’s Role in Asthma
PUFA Decrease Cellular Energy Production
Sunburn, PUFA, Prostaglandins, and Aspirin
Three important kinds of enzymes that are activated by stress and radiation are phospholipases (that release fatty acids), tryptophan hydroxylase (that controls the conversion of tryptophan to serotonin), and aromatase (estrogen synthetase, that converts androgens to estrogen). The products of these enzymes stimulate cell division, and produce features of the inflammatory process, including the leakiness of capillaries. -Ray Peat, PhD
Serotonin itself is toxic to nerves, and is part of the adaptive system that gets out of control during prolonged inflammation. Serotonin is an important activator of the phospholipases. -Ray Peat, PhD
People who take aspirin, drink coffee, and use tobacco, have a much lower incidence of Alzheimer’s disease than people who don’t use those things. Caffeine inhibits brain phospholipase, making it neuroprotective in a wide spectrum of conditions. In recent tests, aspirin has been found to prevent the misfolding of the prion protein, and even to reverse the misfolded beta sheet conformation, restoring it to the harmless normal conformation. Nicotine might have a similar effect, preventing deposition of amyloid fibrils and disrupting those already formed (Ono, et al., 2002). Vitamin E, aspirin, progesterone, and nicotine also inhibit phospholipase, which contributes to their antiinflammatory action. Each of the amyloid-forming proteins probably has molecules that interfere with its toxic accumulation. -Ray Peat, PhD
J Biochem. 2002 Mar;131(3):285-92.
Phospholipase A2.
Murakami M, Kudo I.
Phospholipase A2 (PLA2) catalyzes the hydrolysis of the sn-2 position of membrane glycerophospholipids to liberate arachidonic acid (AA), a precursor of eicosanoids including prostaglandins (PGs) and leukotrienes (LTs). The same reaction also produces lysophosholipids, which represent another class of lipid mediators. So far, at least 19 enzymes that possess PLA2 activity have been identified in mammals. The secretory PLA2 (sPLA2) family, in which 10 isozymes have been identified, consists of low-molecular-weight, Ca2+-requiring, secretory enzymes that have been implicated in a number of biological processes, such as modification of eicosanoid generation, inflammation, host defense, and atherosclerosis. The cytosolic PLA2 (cPLA2) family consists of 3 enzymes, among which cPLA2alpha plays an essential role in the initiation of AA metabolism. Intracellular activation of cPLA2alpha is tightly regulated by Ca2+ and phosphorylation. The Ca2+-independent PLA2 (iPLA2) family contains 2 enzymes and may play a major role in membrane phospholipid remodeling. The platelet-activating factor (PAF) acetylhydrolase (PAF-AH) family represents a unique group of PLA2 that contains 4 enzymes exhibiting unusual substrate specificity toward PAF and/or oxidized phospholipids. In this review, we will overview current understanding of the properties and functions of each enzyme belonging to the sPLA2, cPLA2, and iPLA2 families, which have been implicated in signal transduction.
Crit Rev Immunol. 1997;17(3-4):225-83.
Regulatory functions of phospholipase A2.
Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I.
Phospholipase A2 (PLA2) plays crucial roles in diverse cellular responses, including phospholipid digestion and metabolism, host defense and signal transduction. PLA2 provides precursors for generation of eicosanoids, such as prostaglandins (PGa) and leukotrienes (LTs), when the cleaved fatty acid is arachidonic acid, platelet-activating factor (PAF) when the sn-1 position of the phosphatidylcholine contains an alkyl ether linkage and some bioactive lysophospholipids, such as lysophosphatidic acid (lysoPA). As overproduction of these lipid mediators causes inflammation and tissue disorders, it is extremely important to understand the mechanisms regulating the expression and functions of PLA2. Recent advances in molecular and cellular biology have enabled us to understand the molecular nature, possible function, and regulation of a variety of PLA2 isozymes. Mammalian tissues and cells generally contain more than one enzyme, each of which is regulated independently and exerts distinct functions. Here we classify mammalian PLA2s into there large groups, namely, secretory (sPLA2), cytosolic (cPLA2), and Ca(2+)-independent PLA2s, on the basis of their enzymatic properties and structures and focus on the general understanding of the possible regulatory functions of each PLA2 isozyme. In particular, the roles of type II sPLA2 and cPLA2 in lipid mediator generation are discussed.
C R Seances Soc Biol Fil. 1996;190(4):409-16.
[Diversity of phospholipases A2 and their functions].
[Article in French]
Bereziat G.
Membrane phospholipids not only constitute structural membrane components, they also contain a wealth of biochemical information. They are the source of numerous lipid mediators (prostaglandins, leukotrienes, thromboxane, paf, lysophosphatidic acid and free fatty acids). These lipids act as second messengers inside the cell to modulate enzyme (e.g. PKC and GAP), ion channels (e.g. Ca2+ and K+) or the activity of factors regulating gene expression either at the transcriptional level (e.g. on the TNF alpha gene) or at the post-transcriptional level (e.g. on the GLUT4 transporter). The synthesis of lipid mediators results from the stimulation of phospholipase A2 (PLA2) activities. PLA2 cleaves membrane phospholipids to give rise to lysophospholipids and to free fatty acids from which second messengers are generated. More specifically, PLA2 provides the precursor for the eicosanoids, when the cleaved fatty acid is arachidonic acid, or for PAF, when the sn-1 position of the phospholipid is an alkyl ether linkage. Therefore, PLA2 is a key enzyme in the regulation of lipid mediators of inflammatory process. The purification and cloning of several PLA2s have demonstrated clear differences between secreted and intracellular PLA2. The secreted PLA2s are closely related proteins of low molecular weight (14 kDa) with calcium requirement in the mM range. They contain numerous bonds and retain the same amino-acids at the active site. In mammals, two types of secreted PLA2 have been identified: type I pancreatic PLA2 and type II inflammatory PLA2 which show 70% sequence homology. Recently, two others 14 kDa sPLA2 have been cloned which share also high homologies with type I and type II but contain respectively 6 and 8 disulpide bonds. In contrast, cellular PLA2s have higher molecular weights (40-110 kDa) and are either calcium independent or require microM amounts for activity. Cellular PLA2s preferentially act on sn-2-arachidonoyl phospholipids in vitro whereas sPLA2 do not display such selectivity in vitro. Both cellular and secreted PLA2s are involved in lipid mediator production. Cellular PLA2 can be activated by membrane receptors coupled to G proteins or by tyrosine kinase receptor, through the ras-raf1-MAP kinases network. Cellular PLA2s are thought to be involved in the initial production of lipid mediators after cell activation. Several lines of evidence suggest that secreted PLA2 is involved in the sustained production of lipid mediators in several cell types. These lines of evidence include the decrease in eicosanoid production by antibodies RNA of sPLA2. Furthermore, secreted PLA2s might trigger autocrine loops and proliferation responses through interaction with a specific receptor.
Biochem J. 1994 Aug 1;301 ( Pt 3):721-6.
Fatty acid and phospholipid selectivity of different phospholipase A2 enzymes studied by using a mammalian membrane as substrate.
Diez E, Chilton FH, Stroup G, Mayer RJ, Winkler JD, Fonteh AN.
Previous studies using phospholipid mixed vesicles have demonstrated that several types of phospholipase A2 (PLA2) enzymes exhibit different selectivity for fatty acids at the sn-2 position, for the type of chemical bond at the sn-1 position or for the phosphobase moiety at the sn-3 position of phospholipids. In the present study, we have utilized natural mammalian membranes from U937 monocytes to determine whether two purified 14 kDa PLA2 isoenzymes (Type I, Type II) and a partially purified 110 kDa PLA2 exhibit substrate selectivity for certain fatty acids or phospholipids. In these studies, arachidonic acid (AA) release from membranes was measured under conditions where the remodelling of AA mediated by CoA-independent transacylase (CoA-IT) activity has been eliminated. In agreement with the mixed-vesicle models, AA was the major unsaturated fatty acid hydrolysed from membranes by the 110 kDa PLA2, suggesting that this PLA2 is selective in releasing AA from natural membranes. By contrast, Type I and Type II PLA2s were less selective in releasing AA from phospholipids and released a variety of unsaturated fatty acids at molar ratios that were proportional to the ratios of these fatty acids in U937 microsomal membranes. Examination of AA release from phospholipid classes indicated that all three enzymes released AA from the major AA-containing phospholipid classes (phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositol) of U937 membranes. The 110 kDa PLA2 released AA from phospholipid subclasses in ratios that were proportional to the AA content within phospholipid classes and subclasses of U937 membranes. These data suggested that the 110 kDa PLA2 shows no preference either for the sn-1 linkage or for the sn-3 phosphobase moiety of phospholipids. By contrast, Type I and Type II PLA2s preferentially released AA from ethanolamine-containing phospholipids and appeared to prefer the 1-acyl-linked subclass. Taken together, these data indicate that the 110 kDa PLA2 selectively releases AA from U937 membranes, whereas Type I and Type II PLA2 release a variety of unsaturated fatty acids. Furthermore, the 110 kDa PLA2 releases the same molar ratios of AA from all major phospholipid subclasses, whereas Type I and Type II PLA2s show some specificity for phosphatidylethanolamine when these enzymes are incubated with a complex mammalian membrane substrate.
J Neurotrauma. 1995 Oct;12(5):791-814.
Mediators of injury in neurotrauma: intracellular signal transduction and gene expression.
Bazan NG, Rodriguez de Turco EB, Allan G.
Membrane lipid-derived second messengers are generated by phospholipase A2 (PLA2) during synaptic activity. Overstimulation of this enzyme during neurotrauma results in the accumulation of bioactive metabolites such as arachidonic acid, oxygenated derivatives of arachidonic acid, and platelet-activating factor (PAF). Several of these bioactive lipids participate in cell damage, cell death, or repair-regenerative neural plasticity. Neurotransmitters may activate PLA2 directly when linked to receptors coupled to G proteins and/or indirectly as calcium influx or mobilization from intracellular stores is stimulated. The release of arachidonic acid and its subsequent metabolism to prostaglandins are early responses linked to neuronal signal transduction. Free arachidonic acid may interact with membrane proteins, i.e., receptors, ion channels, and enzymes, modifying their activity. It can also be acted upon by prostaglandin synthase isoenzymes (the constitutive prostaglandin synthase PGS-1 or the inducible PGS-2) and by lipoxygenases, with the resulting formation of different prostaglandins and leukotrienes. Glutamatergic synaptic activity and activation of postsynaptic NMDA receptors are examples of neuronal activity, linked to memory and learning processes, which activate PLA2 with the consequent release of arachidonic acid and platelet-activating factor (PAF), another lipid mediator. Both mediators may exert presynaptic and postsynaptic effects contributing to long-lasting changes in glutamate synaptic efficacy or long-term potentiation (LTP), PAF, a potential retrograde messenger in LTP, stimulates glutamate release. The PAF antagonist BN 52021 competes for receptors in presynaptic membranes and blocks this effect. PAF may also be involved in plasticity responses because PAF leads to the expression of early response genes and subsequent gene cascades. The PAF antagonist BN 50730, selective for PAF intracellular binding, blocks PAF-mediated induction of gene expression. A consequence of neural injury induced by ischemia, trauma, or seizures is an increased release of neurotransmitters, that in turn generates an overproduction of second messengers. Glutamate, a key player in excitotoxic neuronal damage, triggers increased permeation of calcium mediated by NMDA receptors and activation of PLA2 in postsynaptic neurons. NMDA receptor antagonists reduce the accumulation of free fatty acids and elicit neuroprotection in ischemic damage. Increased production of free arachidonic acid and PAF converges to exacerbate glutamate-mediated neurotransmission. These neurotoxic actions may be brought about by arachidonic acid-induced potentiation of NMDA receptor activity and decreased glutamate reuptake. On the other hand, PAF stimulates the further release of glutamate at presynaptic endings. The neuroprotective effects of the PAF antagonist BN 52021 in ischemia-reperfusion are due, at least in part, to an inhibition of presynaptic glutamate release. PAF also induces expression of the inducible prostaglandin synthase gene, and PAF antagonists selective for the intracellular sites inhibit this effect. The PAF antagonist also inhibits the enhanced abundance, due to vasogenic cerebral edema and ischemia-reperfusion damage, of inducible prostaglandin synthase mRNA in vivo. Therefore, PAF, an injury-generated mediator, may favor the formation of other cell injury and inflammation mediators by turning on the expression of the gene that encodes prostaglandin synthase.
Drug Discov Today. 2003 Aug 1;8(15):710-6.
Phospholipase A2 expression in tumours: a target for therapeutic intervention?
Laye JP, Gill JH.
Phospholipase A(2) (PLA(2)) enzymes are involved in lipid metabolism and, as such, are central to several cellular processes. The different PLA(2)s identified to date can be classified into three groups: secreted PLA(2) (sPLA(2)), calcium-independent PLA(2) (iPLA(2)) and calcium-dependent cytosolic PLA(2) (cPLA(2)). In addition to their role in cellular signalling, PLA(2)s have been implicated in diverse pathological conditions, including inflammation, tissue repair and cancer. Elevated levels of sPLA(2) and cPLA(2) have been reported in several tumour types. Here, we summarize the current views on the PLA(2)s, and look at their expression, role in human malignancy and potential as targets for anticancer drug development.
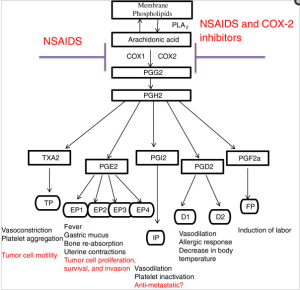
Curr Mol Med. 2001 Dec;1(6):739-54.
Mammalian secreted phospholipases A2 and their pathophysiological significance in inflammatory diseases.
Touqui L, Alaoui-El-Azher M.
Phospholipases A2 (PLA2s) represent a growing family of enzymes that catalyze the hydrolysis of phospholipids at the sn-2 position leading to the generation of free fatty acids and lysophospholipids. Mammalian PLA2s are divided into two major classes according to their molecular mass and location: intracellular PLA2 and secreted PLA2 (sPLA2). Type-IIA sPLA2 (sPLA2-IIA), the best studied enzyme of sPLA2, plays a role in the pathogenesis of various inflammatory diseases. Conversely, sPLA2-IIA can exert beneficial action in the context of infectious diseases since recent studies have shown that this enzyme exhibits potent bactericidal effects. Induction of the synthesis of sPLA2-IIA is generally initiated by endotoxin and a limited number of cytokines via paracrine and/or autocrine processes. If the mechanisms involved in the regulation of sPLA2-IIA gene expression have been relatively clarified, little is known on the mechanisms that regulate the expression of other sPLA2. There have been substantial progresses in understanding the transcriptional regulation of sPLA2-IIA expression. Recently, transcription factors including NF-kappaB, PPAR, C/EBP have been identified to play a prominent role in the regulation of sPLA2-IIA gene expression. The activation of these transcription factors is under the control of distinct signaling pathways (PKC, cAMP …). Accumulating evidences in the literature suggest that cytosolic PLA2 together with two sPLA2 isozymes (sPLA2-IIA and sPLA2-V) are functionally coupled with cyclooxygenase-1 and 2 pathways, respectively, for immediate and delayed PG biosynthesis. This spatio-temporal coupling of cyclooxygenase enzymes with PLA2s may represent a key mechanism in the propagation of inflammatory reaction. Unraveling the mechanisms involved in the regulation of the expression of sPLA2s is important for understanding their pathophysiological roles in inflammatory diseases.
J Biol Chem. 1995 Jun 23;270(25):14855-8.
Nitric oxide activates the glucose-dependent mobilization of arachidonic acid in a macrophage-like cell line (RAW 264.7) that is largely mediated by calcium-independent phospholipase A2.
Gross RW, Rudolph AE, Wang J, Sommers CD, Wolf MJ.
Herein, we demonstrate that nitric oxide is a potent (> 20% release) and highly selective inducer of [3H]arachidonic acid mobilization in the macrophage-like cell line RAW 264.7. Treatment of RAW 264.7 cells with (E)-6-(bromomethylene)-3-(1-naphthalenyl)-2H-tetrahydropyran-2-one resulted in the inhibition of the large majority (86%) of nitric oxide-induced [3H]arachidonic acid release into the medium (IC50 < 0.5 microM) and the concomitant inhibition of in vitro measurable calcium-independent phospholipase A2 activity (92% inhibition) without demonstrable effects on calcium-dependent phospholipase A2 activity. Since nitric oxide is a potent stimulator of glycolysis (and therefore glycolytically derived ATP) and since cytosolic calcium-independent phospholipase A2 exists as a catalytic complex comprised of ATP-modulated phosphofructokinase-like regulatory polypeptides and a catalytic subunit, we examined the role of glucose in facilitating nitric oxide-mediated arachidonic acid release. Nitric oxide-induced release of [3H]arachidonic acid possessed an obligatory requirement for glucose, was highly correlated with the concentration of glucose in the medium, and was dependent on the metabolism of glucose. Thus, [3H]arachidonic acid release is coupled to cellular glucose metabolism through alterations in the activity of calcium-independent phospholipase A2. Collectively, these results identify a unifying metabolic paradigm in which the generation of lipid second messengers is coordinately linked to the signalstimulated acceleration of glycolytic flux, thereby facilitating integrated metabolic responses to cellular stimuli.
Estrogen, by activating phospholipase A2, acts to amplify the toxic effects of PUFA in the tissues, and these effects increase with age, and with decreased amounts of thyroid and progesterone. -Ray Peat, PhD
Estrogen increases lipid peroxidation, and maintains a chronically high circulating level of free fatty acids, mainly PDFA, activates the phospholipases that release arachidonic acid from cells leading to formation of prostaglandins and isoprostanes, and increases the enzymes that form the inflammation-promoting platelet activating factor (PAF) while suppressing the enzymes that destroy it, and increases a broad range of other inflanunatory mediators, interleukins, and NF-kappa B. -Ray Peat, PhD
Steroids. 2006 Mar;71(3):256-65. Epub 2005 Dec 22.
Estrogen induces phospholipase A2 activation through ERK1/2 to mobilize intracellular calcium in MCF-7 cells.
Thomas W, Coen N, Faherty S, Flatharta CO, Harvey BJ.
The principal secreted estrogen, 17beta-estradiol rapidly activates signaling cascades that regulate important physiological processes including ion transport across membranes, cytosolic pH and cell proliferation. These effects have been extensively studied in the MCF-7 estrogen-responsive human breast carcinoma cell line. Here, we demonstrate that a physiological concentration of 17beta-estradiol caused a rapid, synchronous and transient increase in intracellular calcium concentration in a confluent monolayer of MCF-7 cells 2-3 min after treatment. This response was abolished when cells were pre-incubated with the phospholipase A(2) (PLA(2)) inhibitor quinacrine or with the cyclooxygenase inhibitor indomethacin. The translocation of GFP-cPLA(2)alpha to perinuclear membranes occurred 1-2 min after 17beta-estradiol treatment; this translocation was concurrent with the transient phosphorylation of cPLA(2)alpha at serine residue 505. The phosphorylation and translocation of cPLA(2) were sensitive to inhibition of the extracellular signal regulated kinase (ERK) signaling cascade and occurred simultaneously with a transient activation of ERK. The phosphorylation of cPLA(2) could be stimulated by membrane impermeable 17beta-estradiol conjugated to bovine serum albumen and was blocked by an antagonist of the classical estrogen receptor. Here we show, for the first time, that PLA(2) and the eicosanoid biosynthetic pathway are involved in the 17beta-estradiol induced rapid calcium responses of breast cancer cells.
Prostaglandins. 1996 Mar;51(3):191-201.
Effect of hormones and antihormones on phospholipase A2 activity in human endometrial stromal cells.
Periwal SB, Farooq A, Bhargava VL, Bhatla N, Vij U, Murugesan K.
Phospholipase A2 activity was studied in isolated human endometrial predecidual cells, and in human endometrium collected from day 19-23 of the menstrual cycle, by performing a radiochemical assay. Phospholipase A2 activity on day 20 was significantly higher than other days (P < 0.001), and the activity was found to gradually decrease after day 20 of the menstrual cycle. The effects of the hormones estradiol and progesterone, and antihormones tamoxifen and RU 486, were studied on the phospholipase A2 activity in isolated predecidual stromal cells. Estradiol produced a significant stimulatory effect (P < 0.001) on phospholipase A2 activity in predecidual cells, and this effect was antagonized by tamoxifen. The combination of estradiol and tamoxifen was significantly different from estradiol alone (P < 0.001), but not from tamoxifen alone. RU 486 alone significantly increased (P < 0.001) phospholipase A2 activity in predecidual stromal cells. However, progesterone had no effect on phospholipase A2 activity in predecidual stromal cells.
If the cells adapt to the increased calcium, rather than dying, their sensitivity is reduced. This is probably involved in the “defensive inhibition” seen in many types of cell. In the brain, DHA and arachidonic acid “brought the cells to a new steady state of a moderately elevated [intracellular calcium] level, where the cells became virtually insensitive to external stimuli. This new steady state can be considered as a mechanism of self protection” (Sergeeva, et al., 2005). -Ray Peat, PhD
Reprod Nutr Dev. 2005 Sep-Oct;45(5):633-46.
Regulation of intracellular calcium levels by polyunsaturated fatty acids, arachidonic acid and docosahexaenoic acid, in astrocytes: possible involvement of phospholipase A2.
Sergeeva M, Strokin M, Reiser G.
Pathological conditions in the brain, such as ischemia, trauma and seizure are accompanied by increased levels of free n-6 and n-3 polyunsaturated fatty acids (PUFA), mainly arachidonic acid (AA, 20:4n-6) and docosahexaenoic acid (DHA, 22:6n-3). A neuroprotective role has been suggested for PUFA. For investigation of the potential molecular mechanisms involved in neuroprotection by PUFA, we studied the regulation of the concentration of intracellular Ca2+ ([Ca2+]i) in rat brain astrocytes. We evaluated the presence of extracellular PUFA and the release of intracellular PUFA. Interestingly, only the constitutive brain PUFA AA and DHA, but not eicosapentaenoic acid (EPA) had prominent effects on intracellular Ca2+. AA and DHA suppressed [Ca2+]i oscillation, inhibited store-operated Ca2+ entry, and reduced the amplitudes of Ca2+ responses evoked by agonists of G protein-coupled receptors. Moreover, prolonged exposure of astrocytes to AA and DHA brought the cells to a new steady state of a moderately elevated [Ca2+]i level, where the cells became virtually insensitive to external stimuli. This new steady state can be considered as a mechanism of self-protection. It isolates disturbed parts of the brain, because AA and DHA reduce pathological overstimulation in the tissue surrounding the damaged area. In inflammation-related events, frequently AA and DHA exhibit opposite effects. However, in astrocytes AA and DHA exerted comparable effects on [Ca2+]i. Extracellularly added AA and DHA, but not EPA, were also able to induce the release of [3H]AA from prelabeled astrocytes. Therefore, we also suggest the involvement of phospholipase A2 activation and lysophospholipid generation in the regulation of intracellular Ca2+ in astrocytes.
Increasing intracellular calcium activates phospholipases, releasing more polyunsaturated fats (Sweetman, et al., 1995). -Ray Peat, PhD
Arch Biochem Biophys. 1995 Oct 20;323(1):97-107.
Effect of linoleic acid hydroperoxide on endothelial cell calcium homeostasis and phospholipid hydrolysis.
Sweetman LL, Zhang NY, Peterson H, Gopalakrishna R, Sevanian A.
J Biol Chem 1995 Jun 23;270(25):14855-8.
Nitric oxide activates the glucose-dependent mobilization of arachidonic acid in a macrophage-like cell line (RAW 264.7) that is largely mediated by calcium-independent phospholipase A2.
Gross RW; Rudolph AE; Wang J; Sommers CD; Wolf MJ.
Herein, we demonstrate that nitric oxide is a potent (> 20% release) and highly selective inducer of [3H]arachidonic acid mobilization in the macrophage-like cell line RAW 264.7. Treatment of RAW 264.7 cells with (E)-6-(bromomethylene)-3-(1-naphthalenyl)-2H-tetrahydropyran-2-one resulted in the inhibition of the large majority (86%) of nitric oxide-induced [3H]arachidonic acid release into the medium (IC50 < 0.5 microM) and the concomitant inhibition of in vitro measurable calcium-independent phospholipase A2 activity (92% inhibition) without demonstrable effects on calcium-dependent phospholipase A2 activity. Since nitric oxide is a potent stimulator of glycolysis (and therefore glycolytically derived ATP) and since cytosolic calcium-independent phospholipase A2 exists as a catalytic complex comprised of ATP-modulated phosphofructokinase-like regulatory polypeptides and a catalytic subunit, we examined the role of glucose in facilitating nitric oxide-mediated arachidonic acid release. Nitric oxide-induced release of [3H]arachidonic acid possessed an obligatory requirement for glucose, was highly correlated with the concentration of glucose in the medium, and was dependent on the metabolism of glucose. Thus, [3H]arachidonic acid release is coupled to cellular glucose metabolism through alterations in the activity of calcium-independent phospholipase A2. Collectively, these results identify a unifying metabolic paradigm in which the generation of lipid second messengers is coordinately linked to the signal stimulated acceleration of glycolytic flux, thereby facilitating integrated metabolic responses to cellular stimuli.
Proc Natl Acad Sci U S A 1990 Nov;87(22):8845-9.
Incorporation of marine lipids into mitochondrial membranes increases susceptibility to damage by calcium and reactive oxygen species: evidence for enhanced activation of phospholipase A2 in mitochondria enriched with n-3 fatty Acids.
Malis CD, Weber PC, Leaf A, Bonventre JV.
Experiments were designed to evaluate the susceptibility of mitochondrial membranes enriched with n-3 fatty acids to damage by Ca2+ and reactive oxygen species. Fatty acid content and respiratory function were assessed in renal cortical mitochondria isolated from fish-oil- and beef-tallow-fed rats. Dietary fish oils were readily incorporated into mitochondrial membranes. After exposure to Ca2+ and reactive oxygen species, mitochondria enriched in n-3 fatty acids, and using pyruvate and malate as substrates, had significantly greater changes in state 3 and uncoupled respirations, when compared with mitochondria from rats fed beef tallow. Mitochondrial site 1 (NADH coenzyme Q reductase) activity was reduced to 45 and 85% of control values in fish-oil- and beef-tallow-fed groups, respectively. Exposure to Ca2+ and reactive oxygen species enhance the release of polyunsaturated fatty acids enriched at the sn-2 position of phospholipids from mitochondria of fish-oil-fed rats when compared with similarly treated mitochondria of beef-tallow-fed rats. This release of fatty acids was partially inhibited by dibucaine, the phospholipase A2 inhibitor, which we have previously shown to protect mitochondria against damage associated with Ca2+ and reactive oxygen species. The results indicate that phospholipase A2 is activated in mitochondria exposed to Ca2+ and reactive oxygen species and is responsible, at least in part, for the impairment of respiratory function. Phospholipase A2 activity and mitochondrial damage are enhanced when mitochondrial membranes are enriched with n-3 fatty acids.
Serum amyloid A, which can increase 1000-fold under the influence of proinflammatory cytokines, resulting from irradiation, stress, trauma, or infection, is an activator of phospholipase A2 (PLA2), which releases fatty acids. Some of the neurodegenerative states, including amyloid-prion diseases, involve activated PLA2, as well as increases in the toxic breakdown products of the polyunsaturated fatty acids, such as 4-hydroxynonenal. The quantity of PUFA in the tissues strongly determines the susceptibility of the tissue to injury by radiation and other stresses. But a diet rich in PUFA will produce brain damage even without exceptional stressors, when there aren’t enough antioxidants, such as vitamin E and selenium, in the diet. -Ray Peat, PhD
J Biol Chem. 2004 Aug 27;279(35):36405-11. Epub 2004 Jun 21.
Phospholipase A2 inhibitors or platelet-activating factor antagonists prevent prion replication.
Bate C, Reid S, Williams A.
A key feature of prion diseases is the conversion of the cellular prion protein (PrP(C)) into disease-related isoforms (PrP(Sc)), the deposition of which is thought to lead to neurodegeneration. In this study a pharmacological approach was used to determine the metabolic pathways involved in the formation of protease-resistant PrP (PrP(res)) in three prion-infected cell lines (ScN2a, SMB, and ScGT1 cells). Daily treatment of these cells with phospholipase A(2) (PLA(2)) inhibitors for 7 days prevented the accumulation of PrP(res). Glucocorticoids with anti-PLA(2) activity also prevented the formation of PrP(res) and reduced the infectivity of SMB cells. Treatment with platelet-activating factor (PAF) antagonists also reduced the PrP(res) content of cells, while the addition of PAF reversed the inhibitory effect of PLA(2) inhibitors on PrP(res) formation. ScGT1 cells treated with PLA(2) inhibitors or PAF antagonists for 7 days remained clear of detectable (PrPres) when grown in control medium for a further 12 weeks. Treatment of non-infected cells with PLA(2) inhibitors or PAF antagonists reduced PrP(C) levels suggesting that limiting cellular PrP(C) may restrict prion formation in infected cells. These data indicate a pivotal role for PLA(2) and PAF in controlling PrP(res) formation and identify them as potential therapeutic agents.
Zhongguo Yao Li Xue Bao. 1994 Jul;15(4):299-302.
Three drugs inhibit phospholipase A2-induced high permeability of endothelial monolayers.
Chen SF, Li SH, Ding FY.
The permeability of aortic endothelial monolayers to fluid and albumin increased 13.5 and 16.1 times respectively after pretreatment with phospholipase A2 (PLA2, 100 U.ml-1) for 30 min. 1-(p-Chlorobenzoyl)-5-methylindole-3-acetic acid (1.16 mmol.L-1), SRI 63-441 (30 nmol.L-1), and 1,25-dihydroxycholecalciferol (0.1 mumol.L-1) decreased PLA2-induced high permeability. PLA2 did not damage the endothelial cells significantly. Our results indicate that the action of PLA2 to increase the permeability of endothelial monolayers is mainly due to PLA2-induced lipid mediators released from endothelial cells.
Inflammation. 1990 Jun;14(3):267-73.
Phospholipase A2-induced lung edema and its mechanism in isolated perfused guinea pig lung.
Chen SF, Li SH, Fei X, Wu ZL.
Lung injury induced by phospholipase A2 (PLA2, 0.046 IU/ml perfusate) was studied in a continuous weighing system of isolated perfused guinea pig lungs. The results revealed that lung weight increased progressively during the 30-min perfusion of PLA2. No change of pulmonary arterial pressure was observed in the same period. Albumin permeability-surface area product, lung index, lung water content, exudate from pleura, and angiotensin-converting-enzyme activity increased significantly at the end of 30 min PLA2 perfusion. p-Bromophenacyl bromide, a PLA2 inhibitor, may block the above changes nearly completely. The effects of inhibitors of cyclooxygenase (indomethacin, IM), lipoxygenase (diethylcarbamaxine, DE), and platelet-activating factor (SRI 63-441) on PLA2-induced lung injury were also studied. We found: (1) PLA2 may induce high permeability lung edema. The role of endothelial injury in the permeability change remains to be further investigated. (2) DE ameliorated lung injury significantly within 10 min of PLA2 treatment but showed no effect after 15 min. IM ameliorated lung injury during the whole experimental period. SRI 63-441 had no effect. It is suggested that PLA2 may damage lung by inducing products of cyclooxygenase and lipoxygenase besides its direct effect.
Am Rev Respir Dis. 1990 Nov;142(5):1193-9.
Phospholipase A2-induced pulmonary and hemodynamic responses in the guinea pig. Effects of enzyme inhibitors and mediators antagonists.
Tocker JE, Durham SK, Welton AF, Selig WM.
The effect of phospholipase A2 (Naja naja) PLA2) on mean arterial blood pressure and intratracheal pressure was examined in anesthetized guinea pigs. Intracheally administered PLA2 (1 to 10 U) produced acute, dose-dependent increases in mean arterial blood pressure and intracheal pressure. However, Intravenously administered PLA2 (doses as large as 1,000 U) did not alter monitored variables. Acute PLA2-induced morphologic alterations were characterized by airway constriction, airway/alveolar cell damage, and pulmonary sequestration of both leukocytes and platelets. PLA2-induced increases in both mean arterial blood pressure and intratracheal pressure were attenuated to varying degrees by pretreating intravenously with indomethacin (10 mg/kg), a cyclooxygenase inhibitor, and WEB 2086 (0.1 mg/kg), a platelet-activating factor antagonist. Both ICI 198,615 (1 mg/kg), a leukotriene D4, receptor antagonist given intravenously, and dexamethasone (50 mg/kg), a steroidal anti-inflammatory agent given intraperitoneally as a 2-day pretreatment, reduced PLA2-induced increases in intratracheal pressure. Pyrilamine (2 mg/kg), a histamine1-receptor antagonist given intravenously, did not modify PLA2-induced pathophysiologic responses. Guinea pigs exposed to aerosolized PLA2 (100 U/ml) exhibited evidence of increased bronchoalveolar lavage macrophage, leukocyte, and lymphocyte accumulation at 24 h post-PLA2. These studies suggest that in vivo PLA2-induced pathophysiologic changes in the guinea pig involve alterations in resident airway cell populations as well as sequestration and infiltration of inflammatory cells. Both eicosanoids and platelet-activating factor appear to contribute to these PLA2-induced pathophysiologic effects.
Prostaglandins Leukot Essent Fatty Acids. 1990 Mar;39(3):167-75.
The effects of neutrophils and phospholipase A2 on transvascular albumin flux in isolated rabbit lungs.
Littner MR, Lott FD.
In this study, addition of phospholipase A2 (PLA2) to salt-perfused isolated rabbit lungs containing rabbit polymorphonuclear leukocytes leads to an increase in pulmonary capillary permeability. We add 1.5 X 10(8) polymorphonuclear leukocytes to the perfusate. Next, indomethacin is added to the perfusate and 40 units of PLA2 are infused into the pulmonary arterial inflow of the lungs. At the end of the study, a lung sample is removed for measurement of transvascular albumin flux using I125-albumin as a measure of the permeability-surface area product. Control studies demonstrate no increase in transvascular albumin flux. Addition of a dual cyclooxygenase and lipoxygenase inhibitor, BW755C, to the perfusate prevents the increase in transvascular albumin flux. We conclude that PLA2 interacts with polymorphonuclear leukocytes to increase protein permeability. Since PLA2 can release endogenous arachidonic acid and platelet-activating factor from cells, this suggests that release of such products may contribute to an increase in pulmonary capillary permeability from polymorphonuclear leukocytes. The ability of BW755C to prevent the increase suggests the possibility that lipoxygenase products contribute.
Am J Pathol. 1990 Jun;136(6):1283-91.
Phospholipase A2-induced pathophysiologic changes in the guinea pig lung.
Durham SK, Selig WM.
The pathophysiology of lung injury induced by phospholipase A2 (PLA2), a lipolytic enzyme implicated in a variety of pulmonary diseases, was examined in the guinea pig. One hundred microliters of saline or 10 units of PLA2 suspended in saline was given as a bolus injection into either the trachea or jugular vein. Intratracheal pressure and mean arterial blood pressure were continuously monitored. The lungs were examined by light and transmission electron microscopy at 1, 10, and 30 minutes after administration. Pulmonary morphologic and physiologic changes were only observed in animals that received PLA2 via the trachea. Significant increases in peak intratracheal pressure occurred as early as 1 minute after intratracheal PLA2 administration. Morphologic evidence of airway constriction, accompanied by blebbing of the apical cytoplasm of airway epithelium, was also observed at this time. A transient increase in mean arterial blood pressure occurred 5 minutes after challenge. At 10 minutes after intratracheal PLA2, there was marked swelling of airway epithelial cells, pronounced blebbing of the apical cytoplasm, and a resultant decrease in size of the airway lumen. Morphologic changes in alveolar cell populations were initially observed 10 minutes after intratracheal PLA2. Interalveolar septa were hypercellular and multifocally thickened. There was prominent perivascular edema and alveolar spaces contained abundant proteinaceous material and occasional hemorrhage. Ultrastructurally, there was marked cell swelling and fragmentation of type I alveolar epithelium resulting in a denuded basal lamina. Sequestration of neutrophils and eosinophils, many of which lacked secretory granules, within alveolar capillaries was accompanied by aggregates of platelets and was observed in close proximity to injured endothelium. Morphologic changes indicative of cell injury were also observed in type II alveolar epithelium. Similar, but more frequent and severe, morphologic injury occurred 30 minutes after intratracheal PLA2. It is concluded that PLA2 induces pronounced morphologic and physiologic changes in the guinea pig and that the route of administration is important in the development of PLA2-induced lung injury.
Thorax. 2008 Jan;63(1):21-6. Epub 2007 Jun 15.
Plasma phospholipase A2 activity in patients with asthma: association with body mass index and cholesterol concentration.
Misso NL, Petrovic N, Grove C, Celenza A, Brooks-Wildhaber J, Thompson PJ.
BACKGROUND:
Secretory phospholipases A2 (sPLA2) have functions relevant to asthmatic inflammation, including eicosanoid synthesis and effects on dendritic cells and T cells. The aim of this study was to measure sPLA2 activity in patients with stable and acute asthma and to assess potential associations with body mass index (BMI), and plasma cholesterol and vitamin C concentrations.
METHODS:
Plasma sPLA2 activity and concentrations of cholesterol and vitamin C were measured in 23 control subjects and 61 subjects with stable asthma (42 mild to moderate, 19 severe). In addition, sPLA2 activity was measured in 36 patients experiencing acute asthma and in 22 of these patients after recovery from the acute attack.
RESULTS:
sPLA2 activity was not significantly greater in severe (499.9 U; 95% confidence interval (CI) 439.4 to 560.4) compared with mild to moderate asthmatic subjects (464.8; 95% CI 425.3 to 504.3) or control subjects (445.7; 95% CI 392.1 to 499.4), although it was higher in patients with acute asthma (581.6; 95% CI 541.2 to 622.0; p<0.001). Male gender, high plasma cholesterol, increased BMI and atopy were associated with increased sPLA2 activity, while plasma vitamin C was inversely correlated with sPLA2 activity in patients with stable asthma and in control subjects. There were significant interactions between gender and plasma cholesterol and between gender and vitamin C in relation to sPLA2 activity.
CONCLUSIONS:
Plasma sPLA2 may provide a biological link between asthma, inflammation, increased BMI, lipid metabolism and antioxidants. Interactions among these factors may be pertinent to the pathophysiology and increasing prevalence of both asthma and obesity.
Cell Mol Biol (Noisy-le-grand). 2004 Feb;50(1):87-94.
The role of secretory phospholipase A2 in acute chest syndrome.
Kuypers FA, Styles LA.
Acute chest syndrome (ACS) is the leading cause of death in sickle cell disease. Severe ACS often develops in the course of a vasoocclusive crisis (VOC), and frequently involves pulmonary fat embolism. Secretory phospholipase A2 (sPLA2), a potent inflammatory mediator, is elevated in ACS, and sPLA2 levels in serum or plasma predict impending ACS. In addition sPLA2 may play a major role in the actual damage to the lung resulting in a new pulmonary infiltrate on chest radiography, respiratory symptoms, and ultimately alveolar collapse and the impairment of gas exchange. The data indicate that measurement of sPLA2 can be useful in alerting the clinician to patients with impending ACS, and suggest that instituting early therapies based on sPLA2 levels, including inhibition of sPLA2 activity, may be useful to prevent or reduce the clinical morbidity of ACS in sickle cell disease.
Blood. 1996 Mar 15;87(6):2573-8.
Phospholipase A2 levels in acute chest syndrome of sickle cell disease.
Styles LA, Schalkwijk CG, Aarsman AJ, Vichinsky EP, Lubin BH, Kuypers FA.
Acute chest syndrome (ACS) is associated with significant morbidity and is the leading cause of death in patients with sickle cell disease (SCD). Recent reports suggest that bone marrow fat embolism can be detected in many cases of severe ACS. Secretory phospholipase A2 (sPLA2) is an important inflammatory mediator and liberates free fatty acids, which are felt to be responsible for the acute lung injury of the fat embolism syndrome. We measured SPLA2 levels in 35 SCD patients during 20 admissions for ACS, 10 admissions for vaso-occlusive crisis, and during 12 clinic visits when patients were at the steady state. Eleven non-SCD patients with pneumonia were also evaluated. To determine if there was a relationship between sPLA2 and the severity of ACS we correlated SPLA2 levels with the clinical course of the patient. In comparison with normal controls (mean = 3.1 +/- 1.1 ng/mL), the non-SCD patients with pneumonia (mean = 68.6 +/- 82.9 ng/mL) and all three SCD patient groups had an elevation of SPLA2 (steady state mean = 10.0 +/- 8.4 ng/mL; vaso-occlusive crisis mean = 23.7 +/- 40.5 ng/mL; ACS mean = 336 +/- 209 ng/mL). In patients with ACS sPLA2 levels were 100-fold greater than normal control values, 35 times greater than values in SCD patients at baseline, and five times greater than non-SCD patients with pneumonia. The degree of SPLA2 elevation in ACS correlated with three different measures of clinical severity and, in patients followed sequentially, the rise in SPLA2 coincided with the onset of ACS. The dramatic elevation of SPLA2 in patients with ACS but not in patients with vaso-occlusive crisis or non-SCD patients with pneumonia and the correlation between levels of SPLA2 and clinical severity suggest a role for SPLA2 in the diagnosis and, perhaps, in the pathophysiology of patients with ACS.
Blood. 2000 Nov 1;96(9):3276-8.
Secretory phospholipase A(2) predicts impending acute chest syndrome in sickle cell disease.
Styles LA, Aarsman AJ, Vichinsky EP, Kuypers FA.
Acute chest syndrome (ACS) is the leading cause of death in sickle cell disease. Severe ACS often develops in the course of a vaso-occlusive crisis (VOC), but currently there are no predictors for its development. Secretory phospholipase A(2) (sPLA(2)), a potent inflammatory mediator, is elevated in ACS, and previous work suggests that sPLA(2) predicts impending ACS. We prospectively evaluated sPLA(2) concentration during 21 admissions for VOC; 6 of these patients went on to develop ACS. Elevation of sPLA(2) was detected all 6 patients 24 to 48 hours before ACS was clinically diagnosed. Adding the requirement for fever raised the specificity of sPLA(2) to 87% while retaining 100% sensitivity. These data indicate that sPLA(2) can be useful in alerting the clinician to patients with impending ACS. In addition, sPLA(2) may be useful for instituting early therapies to prevent or reduce the clinical morbidity of ACS.
Am J Respir Crit Care Med. 1997 Feb;155(2):421-5.
Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics.
Bowton DL, Seeds MC, Fasano MB, Goldsmith B, Bass DA.
Phospholipases A2 (PLA2) hydrolyze phospholipids resulting in the release of fatty acids including arachidonic acid (AA) and lysophospholipids. AA, in turn, serves as a substrate for the synthesis of leukotrienes which can cause bronchoconstriction and airways edema and appear to be important mediators of clinical asthma. Further, lysophospholipids may be cytotoxic and/or impair the function of surfactant. We examined the release of secretory PLA2 (sPLA2) and AA into the airways after antigen challenge in 16 subjects with allergic asthma. Asthmatic subjects underwent bronchoscopy with bronchoalveolar lavage (BAL) before and after inhaled antigen challenge; in addition, a single BAL, without inhaled antigen, was performed in 10 control subjects. BAL was obtained at 4 h (n = 7), the time of the late asthmatic response (LAR) (n = 5), or 24 h (n = 4) after challenge. There was no difference between normal and asthmatic subjects in either BAL fluid (BALF) sPLA2 activity or AA concentration at baseline. Both sPLA2 and AA increased after antigen challenge (p < 0.01 and 0.05, respectively). These changes were most marked 4 h after challenge (p < 0.03 for both). sPLA2 may play an important role in the generation of AA in patients with asthma.
Allergy. 2000;55 Suppl 61:27-30.
Arachidonic acid metabolism in inflammatory cells of patients with bronchial asthma.
Calabrese C, Triggiani M, Marone G, Mazzarella G.
Over the last few years, the demonstration of beneficial effects of leukotriene receptor antagonists in various forms of asthma has renewed clinical and pharmacologic interest in this class of lipid mediators. Several studies demonstrated an increased biosynthesis of cysteinyl leukotrienes (CysLT) in asthmatic patients. However, the reasons for the dysregulated production of CysLTs in asthmatic patients are not completely defined. An improved method of lipid mediator detection and the availability of cells isolated from human airways (by bronchoalveolar lavage [BAL] and bronchial biopsies) have allowed initial studies to address this issue. Eosinophils retrieved from inflamed airways of asthmatics have a larger arachidonic acid (AA) content than their blood counterpart. The high level of AA in these cells is primarily due to a remodeling of endogenous arachidonate pools with the accumulation of this fatty acid in a triglyceride-associated pool. In addition, elevated levels of a secretory form of phospholipase A2, the key enzyme initiating the cascade of CysLTs, are found in the BAL of asthmatics. Finally, eosinophils isolated from the BAL of asthmatics have an increased expression of LTC4 synthase. The level of expression of this enzyme correlates with the increased amount of CysLTs produced in the airways of these patients. Taken together, these data identify at least two possible mechanisms to explain the excessive CysLT production in asthmatics: 1) an increased content of AA in the glycerolipid pools of inflammatory cells 2) an enhanced activity of key biosynthetic enzymes involved in CysLT synthesis.
J Leukoc Biol. 1996 Dec;60(6):704-9.
Secretory phospholipase A2 activity is elevated in bronchoalveolar lavage fluid after ovalbumin sensitization of guinea pigs.
Sane AC, Mendenhall T, Bass DA.
Arachidonic acid (AA), the precursor of eicosanoids, is released from the sn-2 position of phospholipids by both secretory (sPLA2) and cytosolic phospholipase A2 (cPLA2). Eicosanoids have been shown to contribute to bronchospasm in asthma. We measured the enzymatic activity of sPLA2 and cPLA2 in the bronchoalveolar lavage fluid and cells, respectively, in male Hartley guinea pigs sensitized with ovalbumin. sPLA2 activity was also measured from alveolar macrophages (AM) in culture from unsensitized and sensitized animals. There was an increase in sPLA2 activity and AA content in the lavage fluid following sensitization (18.73 +/- 1.33 to 25.74 +/- 3.22% hydrolysis and 17.97 +/- 12.39 to 44.76 +/- 13.37 pmol AA/mL BAL, mean +/- SD), which remained elevated but without further increase 4 or 24 h after antigen challenge. AM from unsensitized and sensitized-unchallenged animals did not secrete sPLA2 activity in culture for 3 h and therefore do not appear to be the cell source of the sPLA2 activity present in the alveolar lavage fluid following OA sensitization. In contrast to the increase in sPLA2 in lung lavage fluid, Western blotting for cPLA2 from lung lavage cells showed no increase 4 or 24 h after antigen challenge compared with sensitization alone. cPLA2 enzymatic activity of the cytosol fraction of lung lavage cells showed no changes with antigen sensitization or challenge. In summary, intraperitoneal sensitization with ovalbumin in male Hartley guinea pigs caused an increase in both sPLA2 and AA in bronchoalveolar lavage fluid without a need for antigen challenge. The increased sPLA2 enzymatic activity following sensitization may be responsible for the elevation of AA in the bronchoalveolar lavage fluid observed after antigen sensitization.
J Thromb Thrombolysis. 2010 Apr;29(3):276-81. Epub 2009 May 17.
Secretory phospholipase A2 in patients with coronary artery disease.
Lima LM, Carvalho MG, da Fonseca Neto CP, Garcia JC, Sousa MO.
This study investigated the correlation of sPLA2 (secretory phospholipase A2) activity with the atheromatosis extent in subjects with coronary artery disease (CAD) undergoing coronary angiography. We analyzed 123 patients, including 35 subjects with angiographically normal coronary arteries (controls), 31 with mild/moderate atheromatosis (stenosis of 30-70% of the luminal diameter in one or more coronary arteries) and 57 with severe atheromatosis (>70% stenosis). Plasma sPLA2 activity was significantly higher in subjects with severe [127.7 U/ml (102.3-162.7); p < 0.0001] and mild/moderate [112.0 U/ml (100.6-146.9); p < 0.0001] atheromatosis than in controls [19.8 U/ml (15.1-32.1)]. In a multiple logistic regression model, adjusted for age, gender, body mass index, tabagism, hypertension, sedentarism, family history for coronary artery disease, diabetes mellitus, total cholesterol, HDLc, LDLc, triglycerides, high sensitivity C-reactive protein and phospholipase A2, only sPLA2 was observed to be independently associated with severe CAD (>70% of stenosis) (p < 0.0001).
Arterioscler Thromb Vasc Biol. 2005 Apr;25(4):839-46. Epub 2005 Feb 3.
Serum levels of type II secretory phospholipase A2 and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study.
Boekholdt SM, Keller TT, Wareham NJ, Luben R, Bingham SA, Day NE, Sandhu MS, Jukema JW, Kastelein JJ, Hack CE, Khaw KT.
OBJECTIVES:
To study the prospective relationship between serum levels of type II secretory phospholipase A2 (sPLA2) and the risk of future coronary artery disease (CAD) in apparently healthy men and women.
METHODS AND RESULTS:
We conducted a prospective nested case-control study among apparently healthy men and women aged 45 to 79 years. Cases (n=1105) were people in whom fatal or nonfatal CAD developed during follow-up. Controls (n=2209) were matched by age, sex, and enrollment time. sPLA2 levels were significantly higher in cases than controls (9.5 ng/mL; interquartile range [IQR], 6.4 to 14.8 versus 8.3 ng/mL; IQR, 5.8 to 12.6; P<0.0001). sPLA2 plasma levels significantly correlated with age, body mass index, systolic blood pressure, high-density lipoprotein (HDL) cholesterol levels, and C-reactive protein (CRP) levels. Taking into account matching for sex and age and adjusting for body mass index, smoking, diabetes, systolic blood pressure, low-density lipoprotein cholesterol, HDL cholesterol, and CRP levels, the risk of future CAD was 1.34 (1.02 to 1.71; P=0.02) for people in the highest sPLA2 quartile, compared with those in the lowest (P for linearity=0.03).
CONCLUSIONS:
Elevated levels of sPLA2 were associated with an increased risk of future CAD in apparently healthy individuals. The magnitude of the association was similar to that observed between CRP and CAD risk, and both associations were independent.
Increased intracellular calcium activates lipolysis (by phospholipases), producing more free fatty acids, as well as excitation and protein breakdown, and in the brain neurodegenerative diseases, calcium excess contributes to clumping synuclein (Wojda, et al., 2008), an important regulator of cytoskeleton proteins. -Ray Peat, PhD
IUBMB Life. 2008 Sep;60(9):575-90.
Calcium ions in neuronal degeneration.
Wojda U, Salinska E, Kuznicki J.
Neuronal Ca(2+) homeostasis and Ca(2+) signaling regulate multiple neuronal functions, including synaptic transmission, plasticity, and cell survival. Therefore disturbances in Ca(2+) homeostasis can affect the well-being of the neuron in different ways and to various degrees. Ca(2+) homeostasis undergoes subtle dysregulation in the physiological ageing. Products of energy metabolism accumulating with age together with oxidative stress gradually impair Ca(2+) homeostasis, making neurons more vulnerable to additional stress which, in turn, can lead to neuronal degeneration. Neurodegenerative diseases related to aging, such as Alzheimer’s disease, Parkinson’s disease, or Huntington’s disease, develop slowly and are characterized by the positive feedback between Ca(2+) dyshomeostasis and the aggregation of disease-related proteins such as amyloid beta, alfa-synuclein, or huntingtin. Ca(2+) dyshomeostasis escalates with time eventually leading to neuronal loss. Ca(2+) dyshomeostasis in these chronic pathologies comprises mitochondrial and endoplasmic reticulum dysfunction, Ca(2+) buffering impairment, glutamate excitotoxicity and alterations in Ca(2+) entry routes into neurons. Similar changes have been described in a group of multifactorial diseases not related to ageing, such as epilepsy, schizophrenia, amyotrophic lateral sclerosis, or glaucoma. Dysregulation of Ca(2+) homeostasis caused by HIV infection or by sudden accidents, such as brain stroke or traumatic brain injury, leads to rapid neuronal death. The differences between the distinct types of Ca(2+) dyshomeostasis underlying neuronal degeneration in various types of pathologies are not clear. Questions that should be addressed concern the sequence of pathogenic events in an affected neuron and the pattern of progressive degeneration in the brain itself. Moreover, elucidation of the selective vulnerability of various types of neurons affected in the diseases described here will require identification of differences in the types of Ca(2+) homeostasis and signaling among these neurons. This information will be required for improved targeting of Ca(2+) homeostasis and signaling components in future therapeutic strategies, since no effective treatment is currently available to prevent neuronal degeneration in any of the pathologies described here.
J Invest Dermatol. 1993 Jan;100(1):35S-41S.
Mechanisms of UV-induced inflammation.
Hruza LL, Pentland AP.
The inflammation produced by exposure to ultraviolet (UV) light has been well documented clinically and histologically. However, the mechanisms by which mediators induce this clinical response remain poorly defined. It is clear that photochemistry occurring after UV absorption must be responsible for initiating these events. Some of these underlying mechanisms have been defined. After exposure to UV light, the formation of prostaglandins and the release of histamine are increased. In addition to an increase in the quantity of these mediators, an increase in sensitivity of irradiated tissue to agonist stimulation also occurs. This increased sensitivity may cause tissue to respond to agonist levels previously present. Phospholipase activity also increases, making more substrate available for prostaglandin formation. Oxygen radical-induced peroxidation of membrane lipids caused by irradiation may contribute to increased phospholipase activity. Oxygen-free radicals also participate in sunburn cell formation and in UV-induced decreases in Langerhans cell numbers. Several enzymatic and non-enzymatic mechanisms are present in skin for reducing these highly reactive oxygen species.