Also see:
Ray Peat, PhD on the Benefits of the Raw Carrot
Endotoxin-lipoprotein Hypothesis
Endotoxin: Poisoning from the Inside Out
Protective Bamboo Shoots
The effect of raw carrot on serum lipids and colon function
Bowel Toxins Accelerate Aging
Thumbs Up: Fructose
The Truth about Low Cholesterol
Are Happy Gut Bacteria Key to Weight Loss?
“The rate of cholesterol production, and the amount in circulation, tend to be inversely related to systemic inflammation. All of the types of lipoprotein absorb, bind, and help to eliminate endotoxin, for example.” -Ray Peat, PhD
“Cholesterol has a long history as a protectant against many toxins; I think this relates to the fact that people with very low cholesterol have such a high incidence of endotoxin-related symptoms.” -Ray Peat, PhD
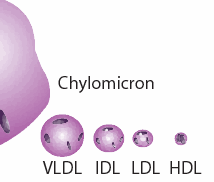
“We are all subject to a variable degree of inflammatory stimulation from the endotoxin absorbed from the intestine, but a healthy liver normally prevents it from reaching the general circulation, and produces a variety of protective factors. The HDL lipoprotein is one of these, which protects against inflammation by binding bacterial endotoxins that have reached the bloodstream. (Things that increase absorption of endotoxin–exercise, estrogen, ethanol–cause HDL to rise.) Chylomicrons and VLDL also absorb, bind, and help to eliminate endotoxins. All sorts of stress and malnutrition increase the tendency of endotoxin to leak into the bloodstream. Thyroid hormone, by increasing the turnover of cholesterol and its conversion into the protective steroids, is a major factor in keeping the inflammatory processes under control.” -Ray Peat, PhD
J Clin Invest. 1993 Mar;91(3):1028-34.
Chylomicrons alter the fate of endotoxin, decreasing tumor necrosis factor release and preventing death.
Harris HW, Grunfeld C, Feingold KR, Read TE, Kane JP, Jones AL, Eichbaum EB, Bland GF, Rapp JH.
The hypertriglyceridemia of infection was traditionally thought to represent the mobilization of substrate to fuel the body’s response to the infectious challenge. However, we have previously shown that triglyceride-rich lipoproteins can protect against endotoxin-induced lethality. The current studies examine the mechanism by which this protection occurs. Rats infused with a lethal dose of endotoxin preincubated with chylomicrons had a reduced mortality compared with rats infused with endotoxin alone (15 vs. 76%, P < 0.001). Preincubation with chylomicrons increased the rate of clearance of endotoxin from plasma and doubled the amount of endotoxin cleared by the liver (30 +/- 1 vs. 14 +/- 2% of the total infused radiolabel, P < 0.001). In addition, autoradiographic studies showed that chylomicrons directed more of the endotoxin to hepatocytes and away from hepatic macrophages. Rats infused with endotoxin plus chylomicrons also showed reduced peak serum levels of tumor necrosis factor as compared with controls (14.2 +/- 3.3 vs. 44.9 +/- 9.5 ng/ml, mean +/- SEM, P = 0.014). In separate experiments, chylomicrons (1,000 mg triglyceride/kg) or saline were infused 10 min before the infusion of endotoxin. Chylomicron pretreatment resulted in a reduced mortality compared with rats infused with endotoxin alone (22 vs. 78%, P < 0.005). Therefore, chylomicrons can protect against endotoxin-induced lethality with and without preincubation with endotoxin. The mechanism by which chylomicrons protect against endotoxin appears to involve the shunting of endotoxin to hepatocytes and away from macrophages, thereby decreasing macrophage activation and the secretion of cytokines.
Infect Immun. 1993 Aug;61(8):3496-502.
Chylomicrons enhance endotoxin excretion in bile.
Read TE, Harris HW, Grunfeld C, Feingold KR, Calhoun MC, Kane JP, Rapp JH.
Chylomicrons prevent endotoxin toxicity and increase endotoxin uptake by hepatocytes. As a consequence, less endotoxin is available to activate macrophages, thereby reducing tumor necrosis factor secretion. To determine whether the chylomicron-mediated increase in hepatocellular uptake of endotoxin results in increased endotoxin excretion into bile, we examined bile after endotoxin administration. A sublethal dose (7 micrograms/kg) of 125I-endotoxin was incubated with either rat mesenteric lymph containing nascent chylomicrons (500 mg of chylomicron triglyceride per kg of body weight) or an equal volume of normal saline (controls) for 3 h and then infused into male Sprague-Dawley rats. Bile samples were collected via a common bile duct catheter for 24 h. Infusion of endotoxin incubated with chylomicrons increased biliary excretion of endotoxin by 67% at 3 h (P < or = 0.006) and by 20% at 24 h (P < or = 0.01) compared with infusion of endotoxin incubated in saline. Endotoxin activity, as measured by the Limulus assay, was not detected in the bile of test animals. However, endotoxin activity was detected after hot phenol-water extraction of bile, demonstrating that endotoxin is inactive in the presence of bile but retains bioactivity after hepatic processing. Since the majority of an intravenous endotoxin load has been shown to be cleared by the liver, acceleration of hepatocyte clearance and biliary excretion of endotoxin may represent a component of the mechanism by which chylomicrons protect against endotoxin-induced lethality.
The Journal of Immunology, 2003, 170: 1399-1405.
Lipopolysaccharide (LPS)-Binding Protein Mediates LPS Detoxification by Chylomicrons 1
Anita C. E. Vreugdenhil, Corine H. Rousseau, Thomas Hartung, Jan Willem M. Greve, Cornelis van ‘t Veer and Wim A. Buurman
Chylomicrons have been shown to protect against endotoxin-induced lethality. LPS-binding protein (LBP) is involved in the inactivation of bacterial toxin by lipoproteins. The current study examined the interaction among LBP, chylomicrons, and bacterial toxin. LBP was demonstrated to associate with chylomicrons and enhance the amount of LPS binding to chylomicrons in a dose-dependent fashion. In addition, LBP accelerated LPS binding to chylomicrons. This LBP-induced interaction of LPS with chylomicrons prevented endotoxin toxicity, as demonstrated by reduced cytokine secretion by PBMC. When postprandial circulating concentrations of chylomicrons were compared with circulating levels of low density lipoprotein, very low density lipoprotein, and high density lipoprotein, chylomicrons exceeded the other lipoproteins in LPS-inactivating capacity. Furthermore, highly purified lipoteichoic acid, an immunostimulatory component of Gram-positive bacteria, was detoxified by incubation with LBP and chylomicrons. In conclusion, our results indicate that LBP associates with chylomicrons and enables chylomicrons to rapidly bind bacterial toxin, thereby preventing cell activation. Besides a role in the detoxification of bacterial toxin present in the circulation, we believe that LBP-chylomicron complexes may be part of a local defense mechanism of the intestine against translocated bacterial toxin.
Ann Surg. 2003 Feb;237(2):246-55.
Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin.
Kono H, Fujii H, Asakawa M, Yamamoto M, Matsuda M, Maki A, Matsumoto Y.
All rats given corn oil died after LPS administration; however, this mortality was prevented by MCT in a dose-dependent manner. Rats given corn oil showed liver injury after LPS administration. In contrast, MCT prevented this pathologic change nearly completely. MCT blunted CD14 expression on the Kupffer cells and TNF-alpha production by isolated Kupffer cells; however, there were no differences in phagocytic index between the two groups. The length of the intestinal epithelium was increased in the MCT group compared to the corn oil group. Further, after LPS administration, increases in gut permeability and injury were prevented by MCT. Importantly, MCT also prevented hepatic energy charge and gut injuries in this condition.
Eur Heart J. 1993 Dec;14 Suppl K:125-9.
The protective effect of serum lipoproteins against bacterial lipopolysaccharide.
Read TE, Harris HW, Grunfeld C, Feingold KR, Kane JP, Rapp JH.
Lipoproteins bind and inactivate bacterial endotoxin, both in vitro and in vivo. Both cholesterol ester-rich and TG-rich lipoproteins, and TG-rich lipid emulsions can prevent death in mice when pre-incubated with a lethal dose of endotoxin before intraperitoneal administration. Chylomicrons can also prevent death when given intravenously after endotoxin in rats. The metabolic fate of lipoprotein-bound endotoxin appears to be directed by the lipoprotein particle. When administered with chylomicrons, the plasma clearance and hepatic uptake of endotoxin are enhanced. Endotoxin is shunted preferentially to hepatocytes and away from hepatic macrophages, thereby increasing endotoxin excretion [corrected] in bile. The survival benefit and alterations in metabolism afforded by chylomicrons correlate with a reduction in peak serum levels of tumour necrosis factor (TNF), providing a possible mechanism by which lipoproteins protect against endotoxin-induced death. These findings suggest a possible role for lipoproteins or lipid emulsions in the body’s defence against endotoxaemia.
Surgery. 1995 Jan;117(1):62-7.
Triglyceride-rich lipoproteins improve survival when given after endotoxin in rats.
Read TE, Grunfeld C, Kumwenda Z, Calhoun MC, Kane JP, Feingold KR, Rapp JH.
Source
Department of Surgery, University of California, San Francisco.
RESULTS:
Chylomicron infusions significantly improved survival when given up to 30 minutes after a lethal dose of endotoxin (p < 0.05). Chylomicrons accelerated endotoxin clearance from the blood and increased endotoxin uptake by the liver. The synthetic triglyceride-rich lipid emulsion significantly improved survival when given up to 15 minutes after a lethal dose of endotoxin (p < 0.05).
CONCLUSIONS:
Triglyceride-rich lipoproteins and synthetic triglyceride-rich lipid emulsions significantly improve survival of rats when given after a lethal dose of endotoxin. Lipoprotein treatment accelerates endotoxin clearance to the liver, which may account for the observed protection. These data suggest a possible therapeutic role for triglyceride-rich lipoproteins or synthetic lipid emulsions in the treatment of the endotoxemia of gram-negative sepsis.
J Exp Med. 1995 Jul 1;182(1):267-72.
Triglyceride-rich lipoproteins prevent septic death in rats.
Read TE, Grunfeld C, Kumwenda ZL, Calhoun MC, Kane JP, Feingold KR, Rapp JH.
Triglyceride-rich lipoproteins bind and inactive bacterial endotoxin in vitro and prevent death when given before a lethal dose of endotoxin in animals. However, lipoproteins have not yet been demonstrated to improve survival in polymicrobial gram-negative sepsis. We therefore tested the ability of triglyceride-rich lipoproteins to prevent death after cecal ligation and puncture (CLP) in rats. Animals were given bolus infusions of either chylomicrons (1 g triglyceride/kg per 4 h) or an equal volume of saline for 28 h after CLP. Chylomicron infusions significantly improved survival (measured at 96 h) compared with saline controls (80 vs 27%, P < or = 0.03). Chylomicron infusions also reduced serum levels of endotoxin, measured 90 min (26 +/- 3 vs 136 +/- 51 pg/ml, mean +/- SEM, P < or = 0.03) and 6 h (121 +/- 54 vs 1,026 +/- 459 pg/ml, P < or = 0.05) after CLP. The reduction in serum endotoxin correlated with a reduction in serum tumor necrosis factor, measured 6 h after CLP (0 +/- 0 vs 58 +/- 24 pg/ml, P < or = 0.03), suggesting that chylomicrons improve survival in this model by limiting macrophage exposure to endotoxin and thereby reducing secretion of inflammatory cytokines. Infusions of a synthetic triglyceride-rich lipid emulsion (Intralipid; KabiVitrum, Inc., Alameda, CA) (1 g triglyceride/kg) also significantly improved survival compared with saline controls (71 vs 27%, P < or = 0.03). These data demonstrate that triglyceride-rich lipoproteins can protect animals from lethal polymicrobial gram-negative sepsis.
J Hepatol. 2004 Sep;41(3):377-83.
High-fat enteral nutrition reduces endotoxin, tumor necrosis factor-alpha and gut permeability in bile duct-ligated rats subjected to hemorrhagic shock.
Luyer MD, Buurman WA, Hadfoune M, Jacobs JA, Dejong CH, Greve JW.
BACKGROUND/AIMS:
Cholestatic patients are prone to septic complications after major surgery due to an increased susceptibility to endotoxin and hypotension. High-fat enteral nutrition reduces endotoxin after hemorrhagic shock. However, it is unknown whether this nutritional intervention is protective in biliary obstruction. We investigated the effect of high-fat enteral nutrition on endotoxin, tumor necrosis factor-alpha (TNF-alpha) and intestinal permeability in cholestatic rats subjected to hemorrhagic shock.
METHODS:
Bile duct-ligated (BDL) rats were fasted or fed with low-fat or high-fat enteral nutrition before hemorrhagic shock. Blood and tissue samples were taken after 90 min.
RESULTS:
Plasma endotoxin decreased after hemorrhagic shock in BDL-rats fed with high-fat nutrition compared to fasted (P<0.01) and low-fat treated rats (P<0.05). Additionally, circulating TNF-alpha was reduced in BDL-rats pretreated with high-fat nutrition compared to fasted rats (P<0.01). The increased intestinal permeability to macromolecules was reduced by high-fat enteral nutrition, whereas bacterial translocation did not significantly change. Simultaneously, tight junction distribution in ileum and colon was disrupted in non-treated BDL-rats but remained unchanged in high-fat pretreated BDL-rats.
CONCLUSIONS:
High-fat enteral nutrition protects against endotoxin-mediated complications independently of intraluminal bile. These results provide a potential new strategy to prevent endotoxin-mediated complications in cholestatic patients undergoing major surgery.
Hepatology. 1997 Dec;26(6):1538-45.
Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol-induced liver disease in the rat.
Nanji AA, Zakim D, Rahemtulla A, Daly T, Miao L, Zhao S, Khwaja S, Tahan SR, Dannenberg AJ.
We investigated the potential of dietary saturated fatty acids to decrease endotoxemia and suppress expression of cyclooxygenase 2 (Cox-2) and tumor necrosis factor alpha (TNF-alpha) in established alcohol-induced liver injury. Six groups (five rats/group) of male Wistar rats were studied. Rats in group 1 were fed a fish oil-ethanol diet for 6 weeks. Rats in groups 2, 3, and 4 were fed fish oil and ethanol for 6 weeks. Ethanol administration was stopped at this time, and the rats were switched to isocaloric diets containing dextrose with fish oil (group 2), palm oil (group 3), or medium-chain triglycerides (group 4) as the source of fat for an additional 2 weeks. Rats in groups 5 and 6 were fed fish oil-ethanol and fish oil-dextrose, respectively, for 8 weeks. Liver samples were analyzed for histopathology, lipid peroxidation, and levels of messenger RNA (mRNA) for Cox-2 and TNF-alpha. Concentrations of endotoxin were determined in plasma. The most severe inflammation and fibrosis were detected in groups 1 and 5, as were the highest levels of endotoxin, lipid peroxidation, and mRNA for Cox-2 and TNF-alpha. After ethanol was discontinued, there was minimal histological improvement in group 2 but near normalization of the histology, including regression of fibrosis, in groups 3 and 4. Histological improvement was associated with decreased levels of endotoxin, lipid peroxidation, and reduced expression of Cox-2 and TNF-alpha. The data indicate that a diet enriched in saturated fatty acids (groups 3 and 4) effectively reverses alcohol-induced liver injury, including fibrosis. The therapeutic effects of saturated fatty acids may be explained, at least in part, by reduced endotoxemia and lipid peroxidation, which in turn result in decreased levels of TNF-alpha and Cox-2.
Critical Care Medicine: April 1996 – Volume 24 – Issue 4 – pp 584-589
Low lipid concentrations in critical illness: Implications for preventing and treating endotoxemia
Gordon, Bruce R. MD; Parker, Thomas S. PhD; Levine, Daniel M. PhD; Saal, Stuart D. MD; Wang, John C. L. MD PhD; Sloan, Betty-Jane MA; Barie, Philip S. MD FCCM; Rubin, Albert L. MD
Objectives: To determine the prevalence and clinical significance of hypolipidemia found in critically ill patients, and whether the addition of a reconstituted lipoprotein preparation could inhibit the generation of tumor necrosis factor-alpha (TNF-alpha) in acute-phase blood taken from these patients.
Setting: Surgical intensive care unit (ICU) of a large urban university hospital.
Design: Prospective case series.
Patients: A total of 32 patients with a variety of critical illnesses had lipid and lipoprotein concentrations determined. Six patients and six age- and gender-matched control subjects had whole blood in vitro studies of the effect of lipoprotein on lipopolysaccharide mediated TNF-alpha production.
Interventions: Blood samples were drawn on admission to the ICU and over a subsequent 8-day period.
Measurements and Main Results: Mean serum lipid and lipoprotein values obtained from patients within 24 hrs of transfer to the surgical ICU were extremely low: mean total cholesterol was 117 mg/dL (3.03 mmol/L), low-density lipoprotein cholesterol 71 mg/dL (1.84 mmol/L), and high-density lipoprotein cholesterol 25 mg/dL (0.65 mmol/L). Only the mean triglyceride concentration of 105 mg/dL (1.19 mmol/L), and the mean lipoprotein(a) concentration of 25 mg/dL (0.25 g/L) were within the normal range. During the first 8 days following surgical ICU admission, there were trends toward increasing lipid and lipoprotein concentrations that were significant for triglycerides and apolipoprotein B. Survival did not correlate with the lipid or lipoprotein concentrations, but patients with infections had significantly lower (p equals .008) high-density lipoprotein cholesterol concentrations compared with noninfected patients. Lipopolysaccharide-stimulated production of TNF-alpha in patient and control blood samples was completely suppressed by the addition of 2 mg/mL of a reconstituted high-density lipoprotein preparation.
Conclusions: Patients who are critically ill from a variety of causes have extremely low cholesterol and lipoprotein concentrations. Correction of the hypolipidemia by a reconstituted high density lipoprotein preparation offers a new strategy for the prevention and treatment of endotoxemia.
Crit Care. 2003; 7(6): 413–414.
Hypocholesterolemia in sepsis and critically ill or injured patients
Robert F Wilson,corresponding author1 Jeffrey F Barletta,2 and James G Tyburski3
Hypocholesterolemia is an important observation following trauma. In a study of critically ill trauma patients, mean cholesterol levels were significantly lower (119 ± 44 mg/dl) than expected values (201 ± 17 mg/dl). In patients who died, final cholesterol levels fell by 33% versus a 28% increase in survivors. Cholesterol levels were also adversely affected by infection or organ system dysfunction. Other studies have illustrated the clinical significance of hypocholesterolemia. Because lipoproteins can bind and neutralize lipopolysaccharide, hypocholesterolemia can negatively impact outcome. New therapies directed at increasing low cholesterol levels may become important options for the treatment of sepsis.
Free Radic Biol Med. 2000 Dec;29(11):1135-42.
Synergistic inhibition of cyclooxygenase-2 expression by vitamin E and aspirin.
Abate A, Yang G, Dennery PA, Oberle S, Schröder H.
The use of aspirin in rheumatoid arthritis is limited since inhibition of the pro-inflammatory enzyme cyclooxygenase-2 occurs only at higher aspirin doses that are often associated with side effects such as gastric toxicity. Using a macrophage cell line (J774. 1A), the present study explores possible synergistic effects of aspirin and vitamin E on the expression and activity of cyclooxygenase-2. Lipopolysaccharide-induced prostaglandin E(2) formation was significantly reduced by aspirin (1-100 microM) or vitamin E (100-300 microM). When combined with vitamin E, aspirin-dependent inhibition of prostaglandin E(2) formation was increased from 59% to 95% of control. Likewise, lipopolysaccharide-induced cyclooxygenase-2 protein and mRNA expression were virtually abolished by the combined treatment of aspirin and vitamin E, whereas the two agents alone were only modestly effective. Vitamin C did not mimic the actions of vitamin E under these conditions, suggesting that redox-independent mechanisms underlie the action of vitamin E. In agreement with this, vitamin E and aspirin were without effect on lipopolysaccharide-induced translocation of the redox-sensitive transcription factor NF-kappa B. Our results show that co-administration of vitamin E renders cyclooxygenase-2 more sensitive to inhibition by aspirin by as yet unknown mechanisms. Thus, anti-inflammatory therapy might be successful with lower aspirin doses when combined with vitamin E, thereby possibly avoiding the side effects of the usually required high dose aspirin treatment.
Infect Immun. 1995 May;63(5):2041-6.
Role for circulating lipoproteins in protection from endotoxin toxicity.
Feingold KR, Funk JL, Moser AH, Shigenaga JK, Rapp JH, Grunfeld C.
Previous studies have shown that endotoxin (lipopolysaccharide [LPS])-induced death can be prevented by preincubating LPS with lipoproteins in vitro or by infusing large quantities of lipids into animals prior to LPS administration. In the present study we determined whether physiological levels of lipids also provide protection. Serum lipid levels were decreased by two different mechanisms: administration of 4-aminopyrolo-(3,4-D)pyrimide, which prevents the hepatic secretion of lipoproteins, and administration of pharmacological doses of estradiol, which increases the number of hepatic low-density lipoprotein receptors, leading to increased lipoprotein clearance. In both hypolipidemic models, LPS-induced mortality is markedly increased compared with that of controls with normal serum lipid levels. In both hypolipidemic models, administration of exogenous lipoproteins, which increase levels of serum lipids into the physiological range, reduces the increased mortality to levels similar to that seen in normal animals. In normal lipidemic animals, 63% of 125I-LPS in plasma is associated with lipoproteins, where it would not be capable of stimulating cytokine production. In contrast, in hypolipidemic animals, very little LPS (12 to 17%) is associated with lipoproteins. Rather, more LPS is in the lipoprotein-free plasma compartment, where it could exert biological effects. In both hypolipidemic models, LPS produces a greater increase in serum tumor necrosis factor levels than it does in controls (three- to fivefold increase), and administration of exogenous lipoproteins prevents this increase. Cytokines, in particular tumor necrosis factor, are responsible for most of the toxic effects of LPS. These data provide evidence that physiological levels of serum lipids protect animals from LPS toxicity. Thus, lipoproteins, in addition to playing a role in lipid transport, may have protective functions. Moreover, as part of the immune response, cytokine-induced increases in serum lipid levels may play a role in host defense by decreasing the toxicities of biological and chemical agents.
J Surg Res. 1999 Apr;82(2):339-45.
Diet-induced protection against lipopolysaccharide includes increased hepatic NO production.
Harris HW, Rockey DC, Young DM, Welch WJ.
The host response to Gram-negative infection includes the elaboration of numerous proinflammatory agents, including tumor necrosis factor alpha (TNFalpha) and nitric oxide (NO). A component of the hepatic response to infection is an elevation in serum lipids, the so-called “lipemia of sepsis,” which results from the increased production of triglyceride (TG)-rich lipoproteins by the liver. We have postulated that these lipoproteins are components of a nonadaptive, innate immune response to endotoxin [lipopolysaccharide (LPS)] and have previously demonstrated the capacity of TG-rich lipoproteins to protect against endotoxicity in rodent models of sepsis. Herein we report the capacity of a high-fructose diet to protect against LPS, most likely by inducing high circulating levels of endogenous TG-rich lipoproteins. The protective phenotype included the increased production of NO by hepatic endothelial cells. Rats, made hypertriglyceridemic by fructose feeding, experienced decreased LPS-induced mortality (P < 0.03) and systemic TNFalpha levels (P < 0.05) as compared with normolipidemic (chow-fed) controls. The increased survival was associated with elevated levels of inducible NO synthase (NOS2) mRNA levels and NO production (82 +/- 26 vs 3 +/- 3 nmol nitrite/10(6) cells, P < 0.001) by hepatic endothelial cells. Nonselective NOS inhibitors reversed the protective phenotype in vivo and readily decreased NO production by cultured endothelial cells from hypertriglyceridemic rats in vitro. This study suggests that a high-fructose diet can protect against endotoxicity in part through induction of endogenous TG-rich lipoproteins and hepatic endothelial cell NO production. This is the first report of diet-induced hyperlipoproteinemia and subsequent protection against endotoxemia.
Am J Physiol. 1999 Nov;277(5 Pt 1):L952-9.
Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine.
Wheeler MD, Thurman RG.
Glycine blunts lipopolysaccharide (LPS)-induced increases in intracellular calcium concentration ([Ca(2+)](i)) and tumor necrosis factor-alpha (TNF-alpha) production by Kupffer cells through a glycine-gated chloride channel. Alveolar macrophages, which have a similar origin as Kupffer cells, play a significant role in the pathogenesis of several lung diseases including asthma, endotoxemia, and acute inflammation due to inhaled bacterial particles and dusts. Therefore, studies were designed here to test the hypothesis that alveolar macrophages could be inactivated by glycine via a glycine-gated chloride channel. The ability of glycine to prevent endotoxin [lipopolysaccharide (LPS)]-induced increases in [Ca(2+)](i) and subsequent production of superoxide and TNF-alpha in alveolar macrophages was examined. LPS caused a transient increase in intracellular calcium to nearly 200 nM, with EC(50) values slightly greater than 25 ng/ml. Glycine, in a dose-dependent manner, blunted the increase in [Ca(2+)](i), with an IC(50) less than 100 microM. Like the glycine-gated chloride channel in the central nervous system, the effects of glycine on [Ca(2+)](i) were both strychnine sensitive and chloride dependent. Glycine also caused a dose-dependent influx of radiolabeled chloride with EC(50) values near 10 microM, a phenomenon which was also inhibited by strychnine (1 microM). LPS-induced superoxide production was also blunted in a dose-dependent manner by glycine and was reduced approximately 50% with 10 microM glycine. Moreover, TNF-alpha production was also inhibited by glycine and also required nearly 10 microM glycine for half-inhibition. These data provide strong pharmacological evidence that alveolar macrophages contain glycine-gated chloride channels and that their activation is protective against the LPS-induced increase in [Ca(2+)](i) and subsequent production of toxic radicals and cytokines.
Am J Physiol Lung Cell Mol Physiol. 2000 Aug;279(2):L390-8.
Dietary glycine blunts lung inflammatory cell influx following acute endotoxin.
Wheeler MD, Rose ML, Yamashima S, Enomoto N, Seabra V, Madren J, Thurman RG.
Mortality associated with endotoxin shock is likely mediated by Kupffer cells, alveolar macrophages, and circulating neutrophils. Acute dietary glycine prevents mortality and blunts increases in serum tumor necrosis factor-alpha (TNF-alpha) following endotoxin in rats. Furthermore, acute glycine blunts activation of Kupffer cells, alveolar macrophages, and neutrophils by activating a glycine-gated chloride channel. However, in neuronal tissue, glycine rapidly downregulates chloride channel function. Therefore, the long-term effects of a glycine-containing diet on survival following endotoxin shock were investigated. Dietary glycine for 4 wk improved survival after endotoxin but did not improve liver pathology, decrease serum alanine transaminase, or effect TNF-alpha levels compared with animals fed control diet. Interestingly, dietary glycine largely prevented inflammation and injury in the lung following endotoxin. Surprisingly, Kupffer cells from animals fed glycine for 4 wk were no longer inactivated by glycine in vitro; however, isolated alveolar macrophages and neutrophils from the same animals were sensitive to glycine. These data are consistent with the hypothesis that glycine downregulates chloride channels on Kupffer cells but not on alveolar macrophages or neutrophils. Importantly, glycine diet for 4 wk protected against lung inflammation due to endotoxin. Chronic glycine improves survival by unknown mechanisms, but reduction of lung inflammation is likely involved.
Lancet. 2000 Sep 9;356(9233):930-3.
The endotoxin-lipoprotein hypothesis.
Rauchhaus M, Coats AJ, Anker SD.
The advent of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) has revolutionised the treatment of hypercholesterolaemia. Statin treatment, by lowering the atherogenic lipoprotein profile, reduces morbidity and mortality in patients with cardiovascular disease. Treatment with simvastatin causes a reduction of events of new-onset heart failure, but this may be attributable to properties other than its lipid-lowering effects. There is some evidence that lower serum cholesterol concentrations (as a surrogate for the totality of lipoproteins) relate to impaired survival in patients with chronic heart failure (CHF). Inflammation is a feature in patients with CHF and increased lipopolysaccharide may contribute substantially. We postulate that higher concentrations of total cholesterol are beneficial in these patients. This is potentially attributable to the property of lipoproteins to bind lipopolysaccharide, thereby preventing its detrimental effects. We hypothesise there is an optimum lipoprotein concentration below which lipid reduction would, on balance, be detrimental. We also propose that, in patients with CHF, a non-lipid-lowering statin (with ancillary properties such as immune modulatory and anti-inflammatory actions) could be as effective or even more beneficial than a lipid-lowering statin.
J Cardiovasc Pharmacol. 2007 Sep;50(3):333-42.
Niacinamide abrogates the organ dysfunction and acute lung injury caused by endotoxin.
Kao SJ, Liu DD, Su CF, Chen HI.
Poly (ADP-ribose) synthabse (PARS) or polymerase (PARP) is a cytotoxic enzyme causing cellular damage. Niacinamide inhibits PARS or PARP. The present experiment tests the effects of niacinamide (NCA) on organ dysfunction and acute lung injury (ALI) following lipopolysaccharide (LPS). LPS was administered to anesthetized rats and to isolated rat lungs. In anesthetized rats, LPS caused systemic hypotension and increased biochemical factors, nitrate/nitrite (NOx), methyl guanidine (MG), tumor necrosis factoralpha (TNFalpha), and interleukin-1beta (IL-1beta). In isolated lungs, LPS increased lung weight (LW) to body weight ratio, LW gain, protein and dye tracer leakage, and capillary permeability. The insult also increased NOx, MG, TNFalpha, and IL-1beta in lung perfusate, while decreased adenosine triphosphate (ATP) content with an increase in PARP activity in lung tissue. Pathological examination revealed pulmonary edema with inflammatory cell infiltration. These changes were abrogated by posttreatment (30 min after LPS) with NCA. Following LPS, the inducible NO synthase (iNOS) mRNA expression was increased. NCA reduced the iNOS expression. Niacinamide exerts protective effects on the organ dysfunction and ALI caused by endotoxin. The mechanisms may be mediated through the inhibition on the PARP activity, iNOS expression and the subsequent suppression of NO, free radicals, and proinflammatory cytokines with restoration of ATP.
Shock. 1998 Dec;10(6):436-41.
Acetazolamide treatment prevents in vitro endotoxin-stimulated tumor necrosis factor release in mouse macrophages.
West MA, LeMieur TL, Hackam D, Bellingham J, Claire L, Rodriguez JL.
We previously showed that incubation in carbon dioxide (CO2), but not air or helium (He), markedly decreased macrophage intracellular pH (pHi) and resulted in reversible inhibition of lipopolysaccharide- (LPS) stimulated tumor necrosis factor (TNF) and interleukin-1 release. We sought to determine whether carbonic anhydrase inhibition with acetazolamide would prevent CO2-mediated inhibition of LPS-stimulated TNF release. Murine peritoneal macrophages were treated with acetazolamide for 1 h under control atmosphere (95% air/5% CO2) and then switched to incubator modules containing: 1) 80% CO2/20% O2, 2) 80% He/20% O2, or 3) 100% air. Before transfer to experimental atmospheric conditions the macrophages were stimulated with 0 or 1 microg/mL of LPS (Escherichia coli 0111 B4). Supernatant TNF was measured 4 h later by bioassay. In parallel experiments LPS-stimulated cytokine mRNA was estimated using reverse transcriptase polymerase chain reaction (RT-PCR) 2 h after LPS stimulation. Viability was determined using dye uptake. Incubation in CO2 or helium had no effect on TNF production in the absence of LPS. In the absence of acetazolamide CO2 produced marked inhibition of LPS-stimulated TNF release, but this was not blocked by the presence of ccc. This CO2-mediated inhibition of TNF was associated with normal levels of TNF mRNA. In acetazolamide-treated macrophages, LPS resulted in a dose-dependent inhibition of TNF release when the cells were incubated in air or helium. Maintenance of normal intracellular pH is required for TNF release, but not TNF mRNA induction by LPS. Factors that alter intracellular pH regulation may modulate LPS-stimulated cytokine production.
Am J Clin Nutr. 2010 Apr;91(4):940-9. doi: 10.3945/ajcn.2009.28584. Epub 2010 Mar 3.
Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression.
Ghanim H1, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, Mohanty P, Dandona P.
Author information
Erratum in
Am J Clin Nutr. 2011 Mar;93(3):674. Upadhyay, Mannish [corrected to Upadhyay, Manish].
Abstract
BACKGROUND:
The intake of glucose or a high-fat, high-carbohydrate (HFHC) meal, but not orange juice, induces an increase in inflammation and oxidative stress in circulating mononuclear cells (MNCs) of normal-weight subjects.
OBJECTIVE:
We investigated the effect of orange juice on HFHC meal-induced inflammation and oxidative stress and the expression of plasma endotoxin and Toll-like receptors (TLRs).
DESIGN:
Three groups (10 subjects in each group) of normal, healthy subjects were asked to drink water or 300 kcal glucose or orange juice in combination with a 900-kcal HFHC meal. Blood samples were obtained before and 1, 3, and 5 h after the drinks and meal combinations were consumed.
RESULTS:
Protein expression of the NADPH oxidase subunit p47(phox), phosphorylated and total p38 mitogen-activated protein kinase, and suppressor of cytokine signaling-3; TLR2 and TLR4 messenger RNA (mRNA) and protein expression; mRNA expression of matrix metalloproteinase (MMP)-9 in MNCs; and plasma concentrations of endotoxin and MMP-9 increased significantly after glucose or water were consumed with the meal but not when orange juice was consumed with the meal. The generation of reactive oxygen species by polymorphonuclear cells was significantly lower when orange juice was added to the meal than when water or glucose was added to the meal.
CONCLUSIONS:
The combination of glucose or water and the HFHC meal induced oxidative and inflammatory stress and an increase in TLR expression and plasma endotoxin concentrations. In contrast, orange juice intake with the HFHC meal prevented meal-induced oxidative and inflammatory stress, including the increase in endotoxin and TLR expression. These observations may help explain the mechanisms underlying postprandial oxidative stress and inflammation, pathogenesis of insulin resistance, and atherosclerosis.
Am J Obstet Gynecol. 2006 Oct;195(4):1015-9.
Progesterone reduces lipopolysaccharide induced interleukin-6 secretion in fetoplacental chorionic arteries, fractionated cord blood, and maternal mononuclear cells.
Gotkin JL1, Celver J, McNutt P, Shields AD, Howard BC, Paonessa DJ, Napolitano PG.
OBJECTIVE:
The purpose of this study was to characterize effect of progesterone (P4) on interleukin-6 (IL-6) production by fetoplacental artery explants, fetal granulocytes, and fetal and maternal mononuclear cells.
STUDY DESIGN:
Arteries and cord blood were obtained from 5 term pregnancies undergoing repeat cesarean section. Maternal blood was obtained from another 6 women at 16 to 20 weeks’ gestation. Tissues were fractionated by dissection or Histopaque gradient. Specimens were incubated in physiologic media then exposed to lipopolysaccharide (LPS) or P4 alone, or pretreated with P4 and then exposed to LPS. Samples were evaluated for IL-6 by enzyme-linked immunosorbent assay (ELISA).
RESULTS:
Arteries and fetal and maternal mononuclear cells exposed to LPS increased IL-6 secretion by 9-, 27-, and 29-fold, respectively. P4 pretreatment blocked LPS induction of IL-6. Fetal granulocytes did not increase IL-6 production in response to LPS exposure.
CONCLUSION:
LPS induces IL-6 in arteries and fetal and maternal mononuclear cells. P4 pretreatment significantly blocks this effect in these cell populations, suggesting possible targets for anti-inflammatory actions of P4 in prevention of preterm birth.

Hi Rob, Thanks for a great web site. I was reading one of your articles about endotoxemia and chylomicrons and the protective effects of cholesterol. I have rheumatoid arthritis and Lyme disease and lately have been suffering with an allergic type reaction to high estrogen. I am also highly endotoxin intolerant, and benefit greatly from taking antibiotics (ostensibly to treat my Lyme, but I think the real benefit may be in decreasing endotoxin and estrogen levels). My question to you is, I have low total cholesterol levels (around 2.5-3.0 in UK measurments). Do you know of any good ways to increase cholesterol levels, preferably with food, but I’d be interested in hearing about supplements too, if there are any. I have 3 other members of my close family with rheumatoid arthritis and we all have low cholesterol levels, so I do feel it is implicated in our disease. Many thanks! Ruth Heasman
Despite the general rule that cholesterol is high in hypothyroidism, cholesterol can be low in hypothyroid individuals when starch consumption is too high, causing excessive endotoxin burden on the liver. Fructose from ripe fruits, fresh OJ, honey, or supplemental fructose can help increase cholesterol production. Excess estrogen, statins, endotoxin, and polyunsaturates are not helpful.
“As far as the evidence goes, it suggests that coconut oil, added regularly to a balanced diet, lowers cholesterol to normal by promoting its conversion into pregnenolone. (The coconut family contains steroids that resemble pregnenolone, but these are probably mostly removed when the fresh oil is washed with water to remove the enzymes which would digest the oil.) Coconut-eating cultures in the tropics have consistently lower cholesterol than people in the U.S. Everyone that I know who uses coconut oil regularly happens to have cholesterol levels of about 160, while eating mainly cholesterol rich foods (eggs, milk, cheese, meat, shellfish). I encourage people to eat sweet fruits, rather than starches, if they want to increase their production of cholesterol, since fructose has that effect.” -Ray Peat, PhD
Without enough cholesterol, estrogen, cortisol, and aldosterone effects are not opposed by protective factors. The blog also provides insight into how it is protective against endotoxin. Thyroid hormone (and vitamin A and light) is involved in the turnover of cholesterol into the opposing steroids like progesterone. Estrogen, PUFA, and endotoxin create self stimulating, inflammatory loops.
Autoimmune Disease and Estrogen Connection
http://www.functionalps.com/blog/2012/03/25/autoimmune-disease-and-estrogen-connection/
J Endocrinol Invest. 1993 Sep;16(8):619-24.
Hormonal pattern in women affected by rheumatoid arthritis.
Valentino R, Savastano S, Tommaselli AP, Riccio A, Mariniello P, Pronesti G, De Divitiis PM, Lombardi G.
Gonadal sex hormones may account for the sexual dimorphism in the immune response and for the greater incidence of autoimmune disease in females. We have previously reported the presence of progesterone (P) deficiency in female patients with thyroid and ovarian autoimmune disease. In this context, the hormonal profile in 9 women with rheumatoid arthritis (RA) and in 9 age-matched ealthy women, were evaluated to verify the presence of a steroid hormone secretion impairment in a systemic autoimmune disease, further supporting our hypothesis of P deficiency involvement. P and androgen plasma levels, in the luteal phase, were significantly lower (p < 0.05 and 0.005, respectively) in RA patients than in the control group, with a consequent decrease of the free androgen index. Moreover, despite normal cortisol values, corticosterone (B) plasma levels were significantly higher in the RA patients (p < 0.01 and 0.05 in follicular and luteal phase, respectively). Therefore, our present data confirm the androgen deficiency in patients with a systemic autoimmune disease, such as RA and support the immunomodulator effect of P. Finally, the higher B plasma levels in RA patients may suggest the presence of a slight impairment of the immune hypothalamic-pituitary-adrenal axis (HPAA), supporting its role in certain phases of RA pathogenesis. In conclusion, in addition to androgens, the immunomodulator role of P should also be taken into account in the pathogenesis of the systemic autoimmune disease. Clin Exp Rheumatol. 2008 Sep-Oct;26(5):903-9. Hydroxylated estrogen metabolites influence the proliferation of cultured human monocytes: possible role in synovial tissue hyperplasia. Capellino S, Montagna P, Villaggio B, Soldano S, Straub RH, Cutolo M. INTRODUCTION: 17Beta-estradiol, estrone, and several of their hydroxylated metabolites, have been found to be significantly increased in synovial fluid of rheumatoid arthritis (RA) patients. In this study, we investigated whether the estrogen metabolites are able to exert direct effects on monocyte cell proliferation, which is important in RA synovial tissue activation and growth. METHODS: Human monocytes (THP-1) were treated with the following estrogen metabolites at different concentrations (from 10-8M, 10-9M, 10-10M to 10-11M) for 24, 48 and 72 hours: 16-hydroxyestrone (16OH-E1), 16-hydroxyestradiol (16OH-E2), 4-hydroxyestrone (4OH-E1), 4-hydroxyestradiol (4OH-E2), 2-hydroxyestrone (2OH-E1) and 2-hydroxyestradiol (2OH-E2). Monocytes were activated with interferon-gamma (INF-gamma). Cell cultures were also performed in presence of tamoxifen (10-7M) to evaluate whether the estrogen metabolites act through the estrogen receptors (ER). Cell growth was detected by MTT test and cell viability through the LDH release assay. RESULTS: 4OH-E1 and 2OH-E1 significantly increased cell growth at low concentration (10-10M), whereas they significantly reduced cell proliferation at high concentrations (10-9M). 16OH-E2 and 4OH-E2 induced opposite effects: cell proliferation at high concentration and antiproliferative action at low doses. On the contrary, 16OH-E1 and 2OH-E2 were found to be estrogen metabolites that induced cell proliferative effects for most of the tested doses. Tamoxifen caused the loss of effects on cell proliferation for almost all the metabolites. CONCLUSION: This study first demonstrates that different downstream estrogen metabolites interfere with monocyte proliferation and generally might modulate the immune response. Therefore, since estrogen metabolite/ratios are altered in the synovial fluid of RA patients, they might play important roles at least in RA synovial tissue hyperplasia. BMJ -VOLUME 303 6 JULY 1991 Sex hormones, autoimmune diseases, and immune response More implications for research than treatment A M DENMAN Autoimmune diseases are far more common in women than in men. For example, the female to male ratio is 9:1 in systemic lupus erythematosus and 4:1 in rheumatoid arthritis.’ These observations suggest that sex hormones may help to determine this susceptibility. Though some research findings seem to confirm this suggestion, others are more equivocal; and more recent work suggests a much more complex role for sex hormones in autoimmune diseases. Some of the most suggestive evidence for the influence of sex hormones relates to rheumatoid arthritis. Thus rheumatoid arthritis begins more commonly in the childbearing years, and both the onset of disease and exacerbations are associated with the postpartum period. Pregnancy is also associated with spontaneous remissions and may itself reduce the risk of developing rheumatoid arthritis. Similarly, autoimmune thyroiditis is encountered as a transient postpartum disorder. Arthritis and Hypothyroidism http://www.functionalps.com/blog/2012/06/23/rheumatoid-arthritis-and-hypothyroidism/
Hormone Balancing: Natural Treatment and Cure for Arthritis
http://www.functionalps.com/blog/2011/10/15/hormone-balancing-natural-treatment-and-cure-for-arthritis/